A chiral organic dye molecule with circularly polarized luminescent properties and its preparation method and application
A chiral separation and cross-coupling reaction technology, applied in the field of dyes, can solve the problems of difficult preparation, limited development of chiral optoelectronic devices, limited application, etc., and achieves the effects of good application prospects, cheap raw materials, and simple synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
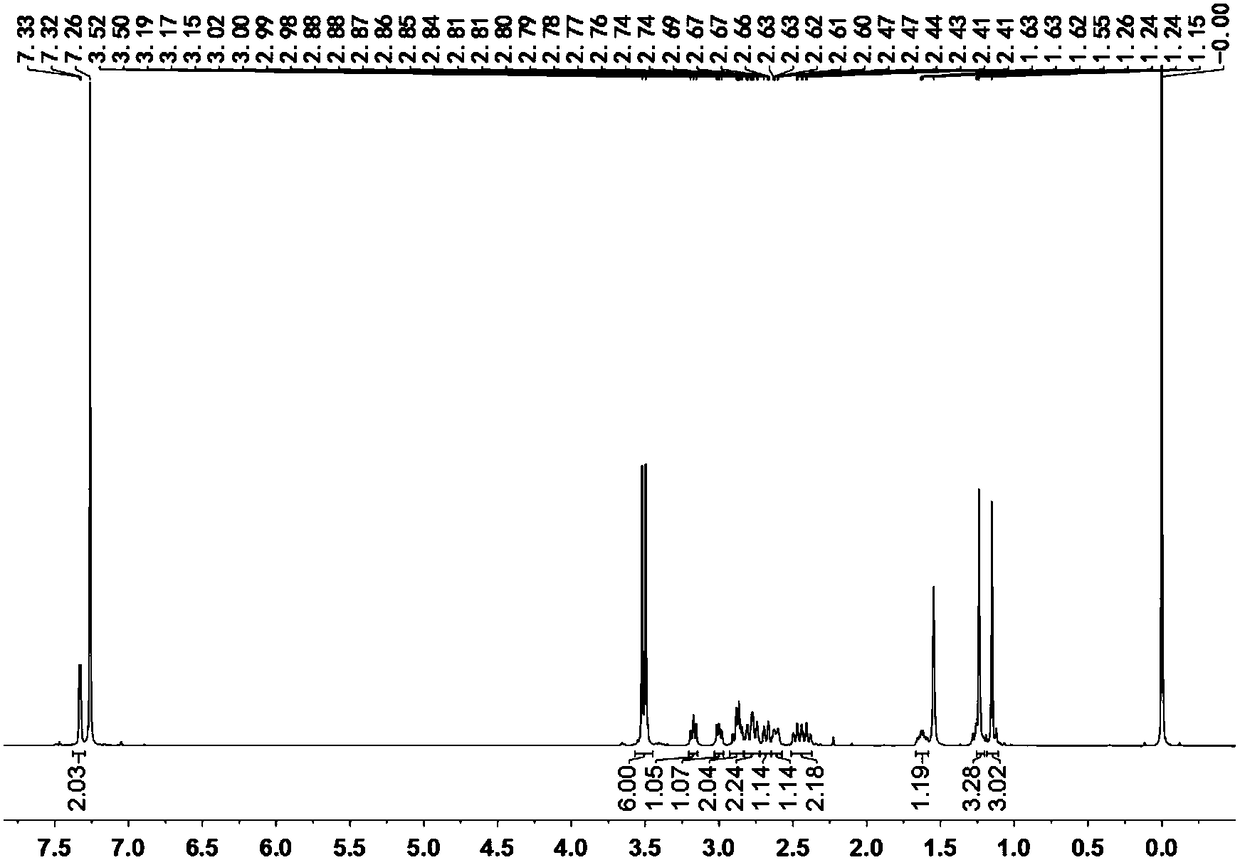
[0086] Embodiment 1, the compound shown in preparation formula D
[0087] The reaction formula is as follows:
[0088]
[0089] 1) Add 159gA, 49g maleic anhydride and 500ml xylene successively in a 1000ml round-bottomed flask, heat and reflux for 10 hours, remove the xylene from the reaction system by steam distillation, after the remaining solid is dried, recrystallize with 500ml acetic anhydride, filter to obtain 87.8 g acid anhydride addition product B, the yield is 82%;
[0090] 2) Add 8.3g of addition product B and 40ml of dichloromethane into a 250ml round bottom flask, dissolve 3ml of liquid bromine in 50ml of acetic acid, put it in a dropping funnel, slowly drop it into the flask at room temperature, and filter it after 12 hours , washed with a small amount of dichloromethane to obtain 9.1 g of oxidative addition product C, with a yield of 80%;
[0091] 3) Add 5.98g (0.01mol) of oxidative addition product C and 5.9g (0.1mol) of n-propylamine to a 250ml round botto...
Embodiment 2
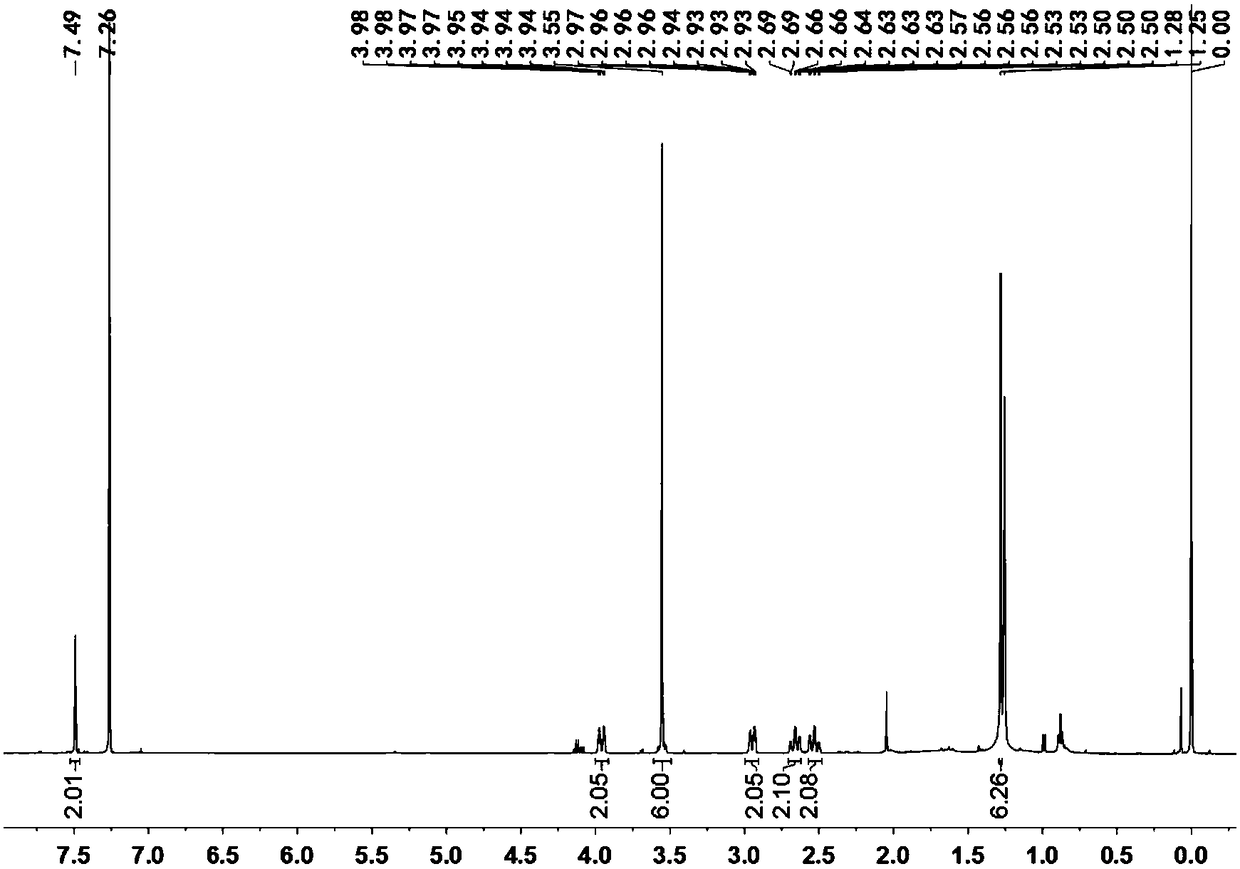
[0097] Embodiment 2, compound shown in preparation formula E and formula F
[0098] The reaction formula is as follows:
[0099]
[0100] 1) Utilize high-performance liquid chromatography (HPLC) chiral column to carry out resolution, and specific chiral column is IC semi-preparative column; mobile phase is CO 2 / MeOH / DCM=40 / 30 / 30 (v / v / v); resolution gave E (e.e. >99%) and F (e.e. >99%).
[0101] The high-performance liquid chromatography data of this compound are shown in table 1 and table 2, and relevant spectrogram sees Figure 7 with Figure 8 Shown:
[0102] Table 1, HPLC data of E
[0103] compound
Ret. Time
Area
Area%
T.Plate#
Height
Height%
E
2.613
8805505
100.00
993952
100.00
2.613
[0104] Table 2, HPLC data of F
[0105]
[0106] From the above detection results, it can be seen that the obtained chiral compound has a correct structure.
Embodiment 3
[0107] Embodiment 3, preparation of the formula G of the compound shown in the attributable formula G 1 Compound shown
[0108] The reaction formula is as follows:
[0109]
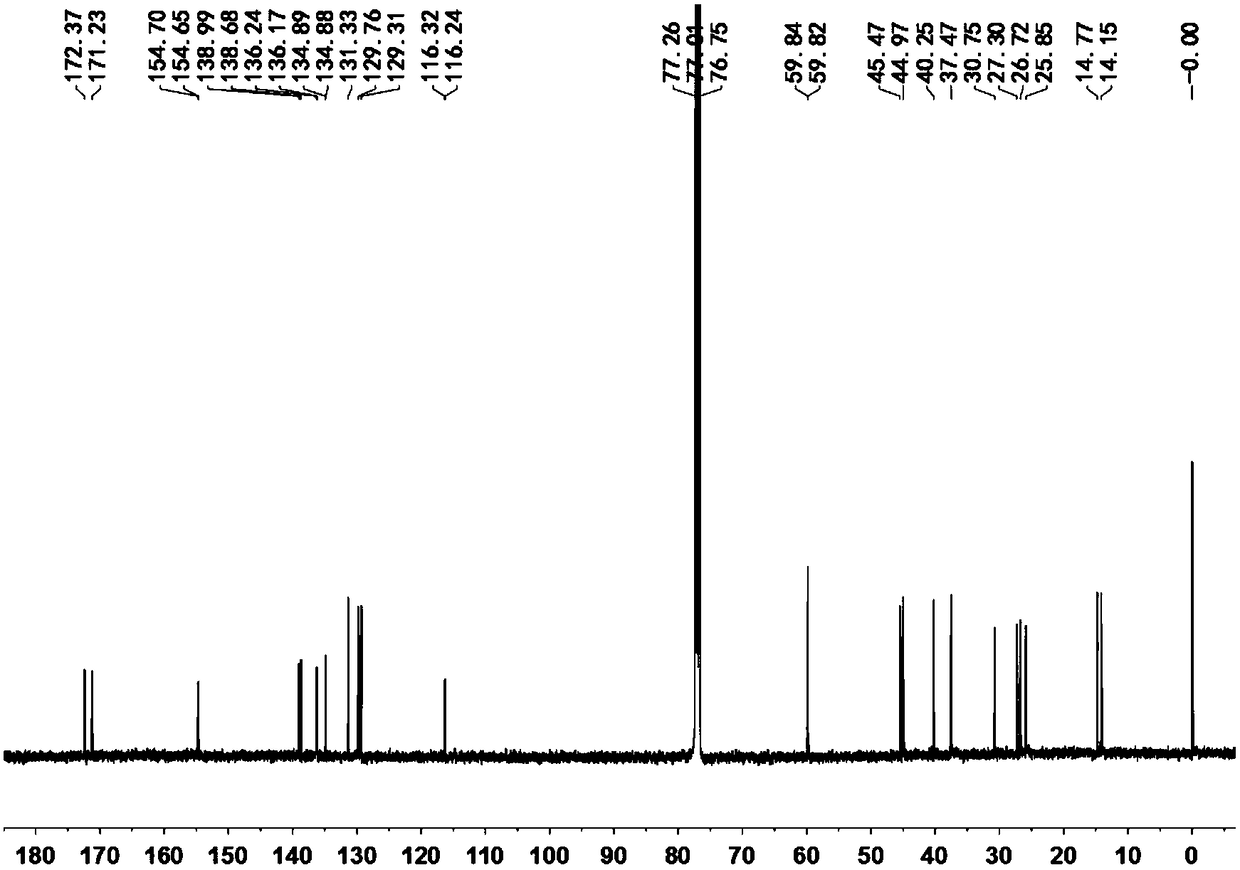
[0110] Add 61mgE and 57mg p-trifluoromethylbenzeneboronic acid into a 25ml two-necked bottle, add 5ml toluene, 3ml ethanol and 2ml 2mol / LNa with a syringe under the protection of argon 2 CO 3 Aqueous solution, add catalyst tetrakis (triphenylphosphine) palladium 5mg after ventilating for 5 minutes, reflux at 100°C for 12 hours, take the organic layer, MgSO 4 Dried, filtered, spin-dried, separated by column chromatography to obtain the G substituted by trifluoromethylphenyl 1 44mg.
[0111] The structure detection result of this compound is as follows:
[0112] 1 H NMR (500MHz, CDCl 3 )δ7.75–7.61(m,8H),7.27(s,2H),4.19–4.14(m,2H),3.73–3.64(m,2H),3.20(s,6H),3.02–2.98(m, 2H), 2.73(td, J=14.6, 3.9Hz, 2H), 2.53(td, J=15.3, 3.9Hz, 2H), 1.75(h, J=7.4Hz, 2H), 1.33(s, 6H), 1.00(t,J=7.4Hz,3H). 13 C NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com