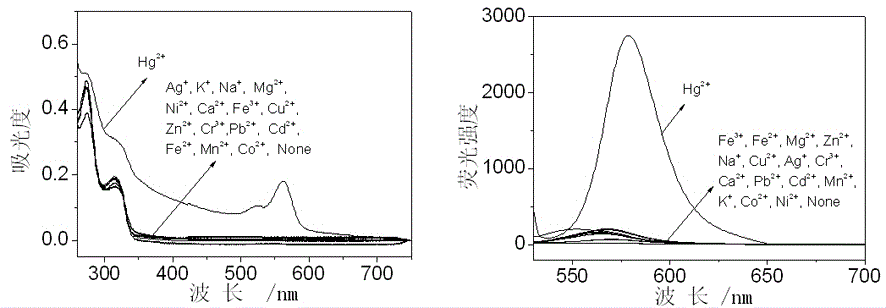
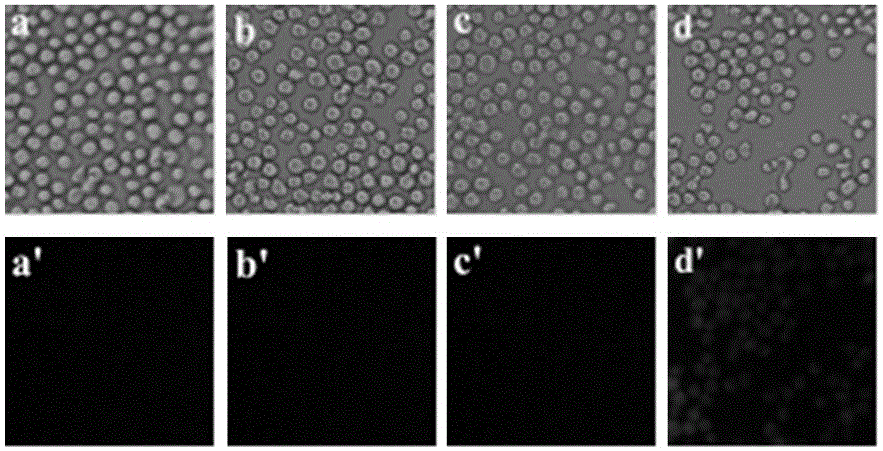
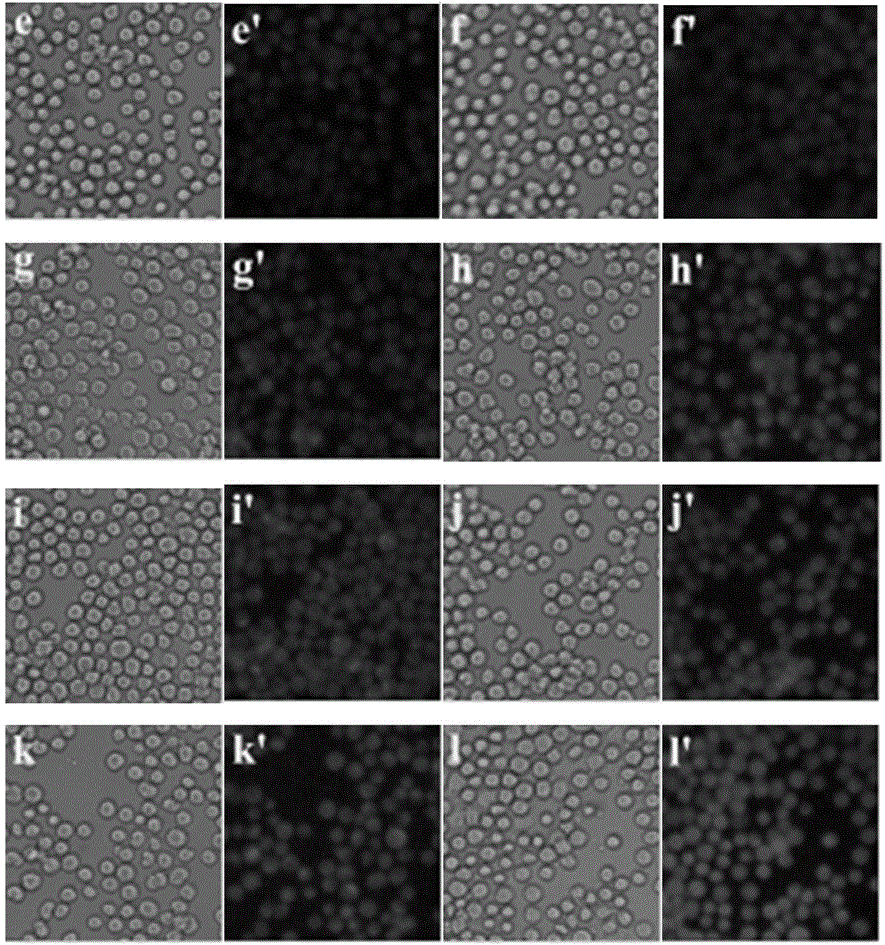
Application of compounds based on rhodamine B and thiobisethylamine in live cell imaging
An aminoethyl sulfide and compound technology, applied in the field of live cell imaging, can solve the problems of less live cell imaging, difficult synthesis, complex structure, etc., and achieve the effects of low toxicity, high sensitivity, high sensitivity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment one: Hg 2+ Preparation of probe RMTE
[0030] The molar ratio of rhodamine B and aminoethyl sulfide is 1:3, with dichloromethane as solvent, N,N-diisopropylethylamine as additive, and the molar ratio of rhodamine B is 7:1, in N 2 Under protection, the reaction was stirred at 30 °C for 20 h, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain the crude product as a yellow solid powder with a yield of 49.8%. Carry out column chromatography separation, eluent is methanol / chloroform / petroleum ether, 1 / 12 / 2 (v / v / v), dry, obtain yellow solid powder target product RMTE, yield rate is 35.1%.
[0031] NMR, 1 H NMR (400 MHz, CDCl 3 ), δ / ppm: 1.16 ( t , 12H, J=6.8 Hz), 2.23( t , 2H, J=8 Hz), 2.50 ( t , 2H, J=6.4 Hz), 2.77 ( t , 2H, J=6.4 Hz), 3.27–2.37 ( m , 10H), 3.66 (s, 2H), 6.27 ( d , 2H, J=6.4 Hz), 6.37 ( d , 2H, J=2.4Hz), 6.44 ( s , 2H), 7.08–7.10 ( m , 1H), 7.43–7.46 ( m , 2H), 7.89–7.91( m , 1H), 13 C NMR (300 ...
Embodiment 2
[0032] Embodiment two: Hg 2+ Preparation of probe RMTE
[0033] The molar ratio of rhodamine B and aminoethyl sulfide is 1:7, with ethanol as solvent, N,N-diisopropylethylamine as additive, and the molar ratio of rhodamine B is 7:1, in N 2 Under protection, the reaction was stirred at 40 °C for 24 h, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain a crude yellow solid powder with a yield of 63.2%. Carry out column chromatography separation, eluent is methanol / chloroform / petroleum ether, 1 / 12 / 2 (v / v / v), dry, obtain yellow solid powder target product RMTE, yield rate is 42.8%.
Embodiment 3
[0034] Embodiment three: Hg 2+ Preparation of probe RMTE
[0035] The molar ratio of rhodamine B and aminoethyl sulfide is 1:5, acetonitrile is used as solvent, N,N-diisopropylethylamine is used as additive, and the molar ratio of rhodamine B is 7:1. 2 Under protection, the reaction was stirred at 20 °C for 18 h, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain the crude product as a yellow solid powder with a yield of 49.8%. Carry out column chromatography separation, eluent is methanol / chloroform / petroleum ether, 1 / 12 / 2 (v / v / v), dry, obtain yellow solid powder target product RMTE, yield rate is 36.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com