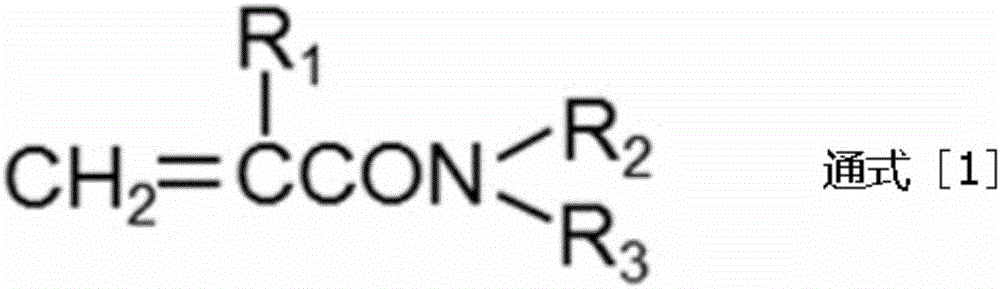
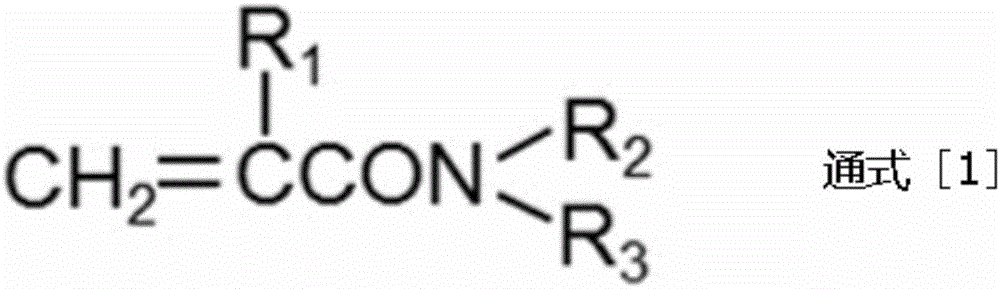Urethane oligomer and active energy ray curable resin composition containing same
An active energy ray, polyurethane technology, used in polyurea/polyurethane adhesives, polyurea/polyurethane coatings, adhesive types, etc., can solve the problems of rising product costs, complicated processes, etc., and achieve high elongation. , The effect of excellent surface curability and excellent chemical resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0090] Hereinafter, the present invention will be more specifically described based on synthesis examples and evaluation examples, but the present invention is not limited to these examples. The physical property analysis of the obtained polyurethane oligomer can be carried out by the following method.
[0091] (1) Molecular weight determination
[0092] The weight-average molecular weight of the obtained polyurethane oligomer and the content of low molecular weight components were determined by high-speed liquid chromatography (chromatography) (manufactured by Shimadzu Corporation "LC-10A", column: Shodex GPC KF-806L (excluding Limit molecular weight: 2×10 7 , Separation range: 100~2×10 7 , number of theoretical plates: 10,000 plates / column, filler material: styrene-divinylbenzene copolymer, filler particle size: 10 μ m), eluent: tetrahydrofuran), and the standard polystyrene molecular weight Calculated.
[0093] (2) Viscosity measurement
[0094] Using a cone-plate visc...
Embodiment B-1
[0168] 100 parts by weight of polyurethane (meth)acrylamide oligomer UTB-1 obtained in Synthesis Example 1, 100 parts by weight of methyl ethyl ketone (MEK) and 3 parts by weight of Darocur 1173 as a photopolymerization initiator were uniformly The mixture was mixed to prepare an active energy ray-curable resin composition. Thereafter, an active energy ray cured film was produced by the following method using the obtained curable resin composition.
[0169] Production method of active energy ray cured film
[0170]Primer coated with a coating bar (RDS 12) on a polyethylene terephthalate (PET) film ("Cosmoshine A4100" manufactured by Toyobo Co., Ltd., single-sided anchor coat treatment) with a thickness of 100 μm On the surface, a coating film was prepared so that the thickness of the dry coating film became 10 μm. After drying the obtained coating film at 80° C. for 2 minutes with an explosion-proof dryer, UV irradiation (device: spot irradiation SUPERCURE-204S manufactured ...
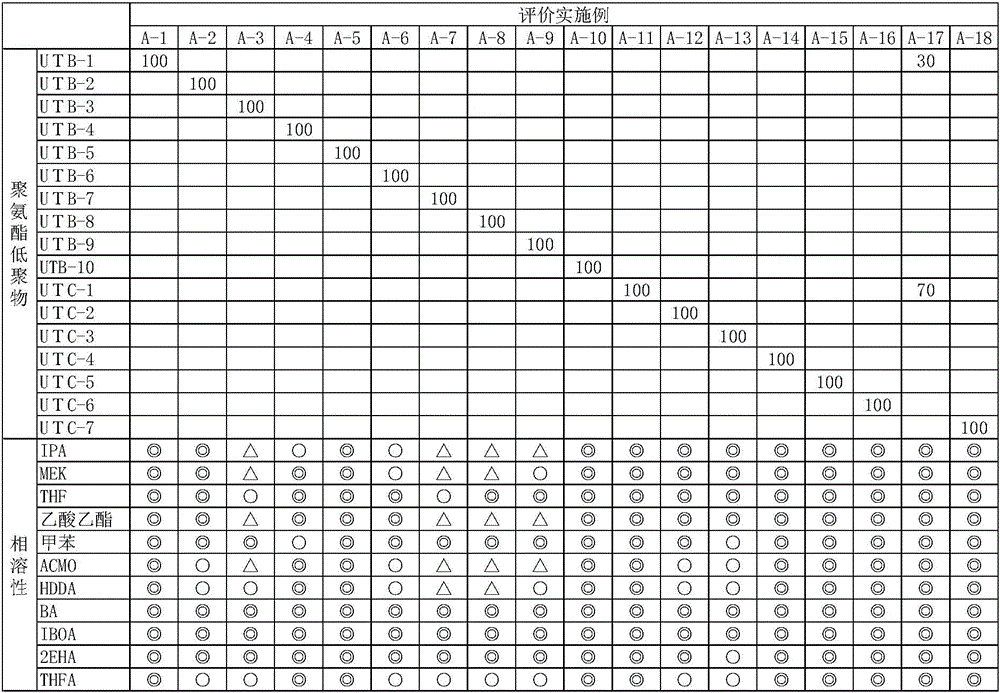
Embodiment B-2~B18、 comparative Embodiment B-19~B-24
[0201] Except having changed into the composition described in Table 3, it carried out similarly to Example B-1, prepared the active energy ray-curable resin composition, produced the cured film, and evaluated it by the said method. The results are shown in Tables 3 and 4.
[0202] [table 3]
[0203]
[0204] [Table 4]
[0205]
[0206] As shown in the results of evaluation examples and evaluation comparative examples, polyurethane oligomers containing 5% by weight or more of low-molecular-weight components require a lot of time and energy to cure, and the cured products obtained have poor adhesion resistance, shrinkage resistance, and water absorption. Difference. The inventors of the present invention think that the reason is that the low molecular weight component is a high polarity component, and since the low molecular weight component is contained, the polarity of the polyurethane oligomer increases as a whole, and the adhesiveness decreases. The content rate of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com