Active vaccine, and preparation method and application thereof
A technology of live vaccines and genes, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve problems such as serious epidemics, mixed infections, and economic losses in the poultry industry, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
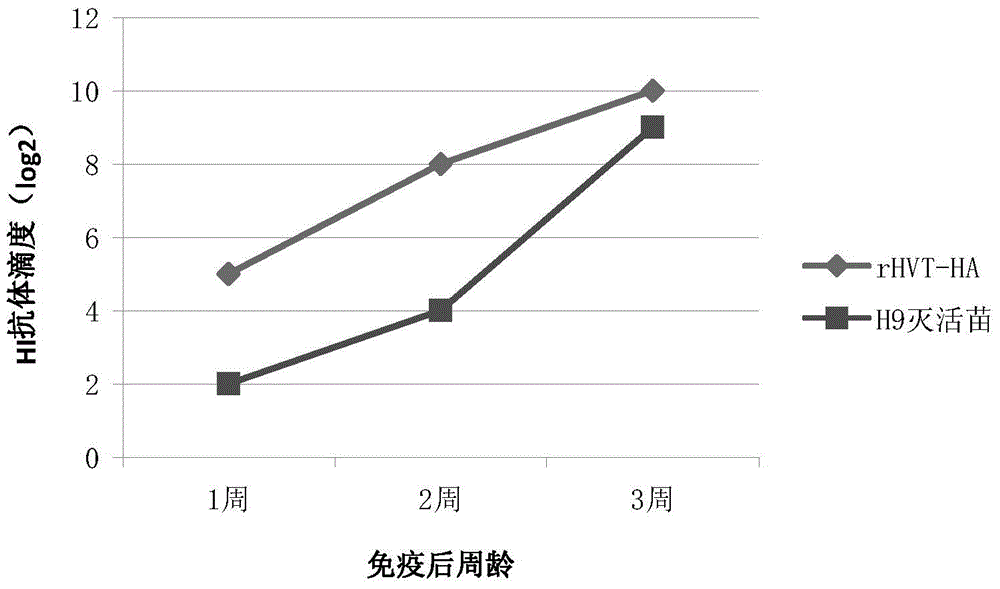
Image
Examples
Embodiment 1
[0049] The construction of embodiment 1 transfer vector
[0050] 1. Extraction of H9 subtype avian influenza virus genome RNA: Inoculate H9 subtype AIVA155-1-2014 strain into 10-day-old SPF chicken embryos, collect the allantoic fluid of dead chicken embryos within 24 to 72 hours, and use Trizol reagent for urine extraction. Extraction of total RNA from cyst fluid. The specific operation was carried out according to the instructions of the reagents.
[0051] 2. RT-PCR: Design a pair of primers, the sequence of which is: upstream primer: 5'-GAAGCGGCCGCAAAATGGAGACAATATCACTAATAAC-3', downstream primer: 5'-GGTGGGCCCTTATACAAATGTTGCATCTGC-3'. Not I and Apa I were introduced at the 5' end of the upstream primer and downstream primer respectively, and the length of the amplified fragment was expected to be 1700bp, and then cloned into the T vector, and the positive recombinant plasmid containing the HA gene was identified by restriction analysis and PCR.
[0052] 3. Construction of ...
Embodiment 2
[0056] Construction, screening and identification of embodiment 2 recombinant Marek's virus
[0057] Chicken embryo fibroblasts (CEF) were prepared according to conventional methods, and after they grew into 80% monolayer, they were inoculated with HVT, the dose was 50 PFU per well in a 24-well plate, and after acting at 37°C for 4 hours, they were treated with Lipofectamine TM 2000 instructions, transfect the cells with the transfer vector plasmid, after 2 hours of action, pour off the transfection solution, add MEM culture solution containing 5% fetal bovine serum, and incubate at 37°C CO 2 After culturing in the incubator for 4 to 5 days, when typical cell lesions appear, add a nutrient solution containing X-Gal (200ug / ml), observe blue plaques, digest the infected CEF cells in 24 wells with 0.25% trypsin, Seed onto 96-well culture plates with fresh CEF cell monolayers. Observe the blue spots, digest the cells with blue holes and connect them to new cells, repeat this cycl...
Embodiment 3
[0061] Embodiment 3 Identification of recombinant virus expressing HA protein
[0062] 1. Indirect immune enzyme reaction Infect the CEF cells in 96 wells with the recombinant virus (rHVT-HA), 4-5 days later. Pour off the culture medium and fix the cells with pre-cooled 95% ethanol and acetone (4:6) mixture; perform immunoenzyme reaction with SPF chicken anti-H9N2 serum and HRP-labeled rabbit anti-chicken IgY.
[0063] Results: After staining with X-Gal, the plaques of CEF cells containing the recombinant virus were blue. The results of immunoenzyme reaction showed that the coeruleus formed by the recombinant virus could specifically react with H9N2 serum.
[0064] 2. Western-blot detection Infect CEF cells with the recombinant virus (rHVT-HA). After 4-5 days, the collected cells were lysed, and the supernatant was taken for 12% SDS-PAGE electrophoresis, and then transferred to the membrane. Anti-H9N2 serum and HRP-labeled rabbit anti-chicken IgY were analyzed by Western-blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com