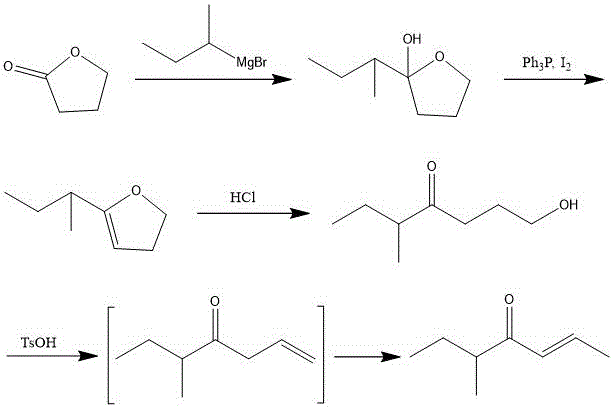
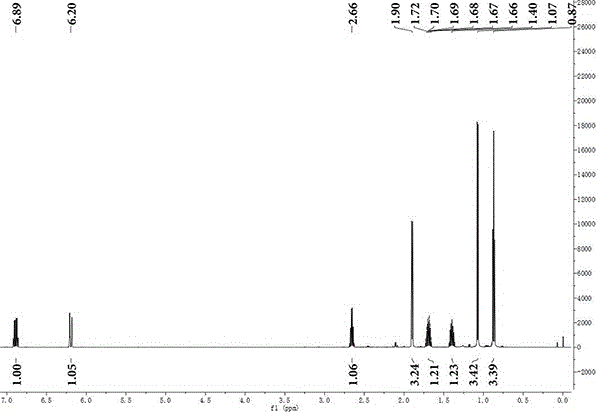
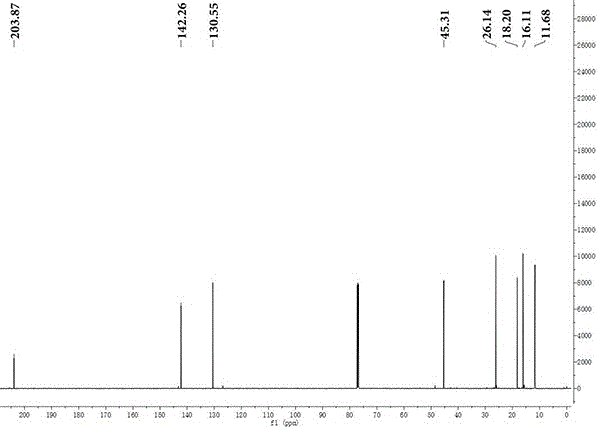
Preparation method of 5-methyl-2-heptylene-4-one
A technology of methyl and heptene, which is applied in the field of preparation of 5-methyl-2-hepten-4-one, can solve the problems of cumbersome operation, long reaction time, and difficulty in industrial production, and avoid oxidation reaction, operate Simple, pollution-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Preparation of 2-sec-butyl-2-hydroxytetrahydrofuran:
[0038] In a 500mL three-necked flask equipped with stirring, condenser, thermometer and dropping funnel, add 0.3mol / 7.2g of Mg activated by hydrochloric acid, two grains of iodine, then add 60mL of tetrahydrofuran dehydrated, and then add 34.3g of 2-bromobutyl Mixed solution of alkane (0.25mol) and 150mL tetrahydrofuran 30mL mixed solution, add the remaining part after triggering, keep slight boiling, reflux for 2 hours after addition, after the reaction is complete, add 17.3g γ-butyrolactone (0.2mol) and 50mL Mixed solution of tetrahydrofuran, keep slightly boiling, reflux for 2 hours after addition, after reaction is complete, add saturated NH 4 Quenched by Cl, extracted with ethyl acetate three times, washed with saturated NaCl, washed with water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by column chromatography to obtain 21 g of 2-sec-butyl-2-hydroxytetrahy...
Embodiment 2
[0039] Embodiment 2: Preparation of 2-sec-butyl-2-hydroxytetrahydrofuran:
[0040] In a 1000mL three-necked flask equipped with stirring, condenser, thermometer and dropping funnel, add Mg0.6mol / 14.4g activated by hydrochloric acid, two grains of iodine, then add 100mL of dehydrated tetrahydrofuran, and then add 68.5g of 2-bromobutyl Mixed solution of alkane (0.5mol) and 300mL tetrahydrofuran 60mL mixed solution, add the remaining part after triggering, keep slightly boiling, reflux for 2 hours after addition, after the reaction is complete, add 34.5g γ-butyrolactone (0.4mol) and 100mL Mixed solution of tetrahydrofuran, keep slightly boiling, reflux for 2 hours after addition, after reaction is complete, add saturated NH 4 Quenched by Cl, extracted 3 times with ethyl acetate, washed with saturated NaCl, washed with water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by column chromatography to obtain 40.4 g of 2-sec-butyl-2-hydroxytetr...
Embodiment 3
[0041] Embodiment 3: Preparation of 2-sec-butyl-2-hydroxytetrahydrofuran:
[0042]In a 2000mL three-necked flask equipped with stirring, condenser, thermometer and dropping funnel, add Mg1.2mol / 28.8g activated by hydrochloric acid, two grains of iodine, then add 200mL of tetrahydrofuran dehydrated, and then add 137g of 2-bromobutane (1mol) and 500mL tetrahydrofuran mixed solution 80mL mixed solution, add the remaining part after triggering, keep a slight boil, reflux for 2 hours after the addition, after the reaction is complete, add dropwise a mixture of 69g γ-butyrolactone (0.8mol) and 150mL tetrahydrofuran Solution, keep slightly boiling, reflux for 2 hours after addition, after the reaction is complete, add saturated NH 4 Quenched by Cl, extracted with ethyl acetate three times, washed with saturated NaCl, washed with water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by column chromatography to obtain 81 g of 2-sec-butyl-2-hydroxytet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com