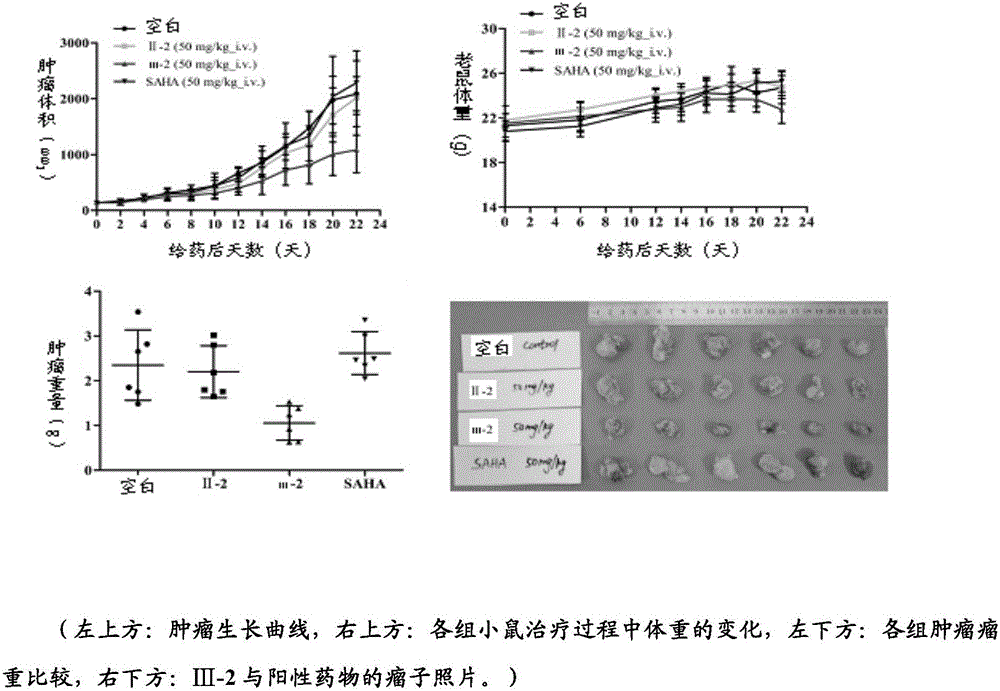
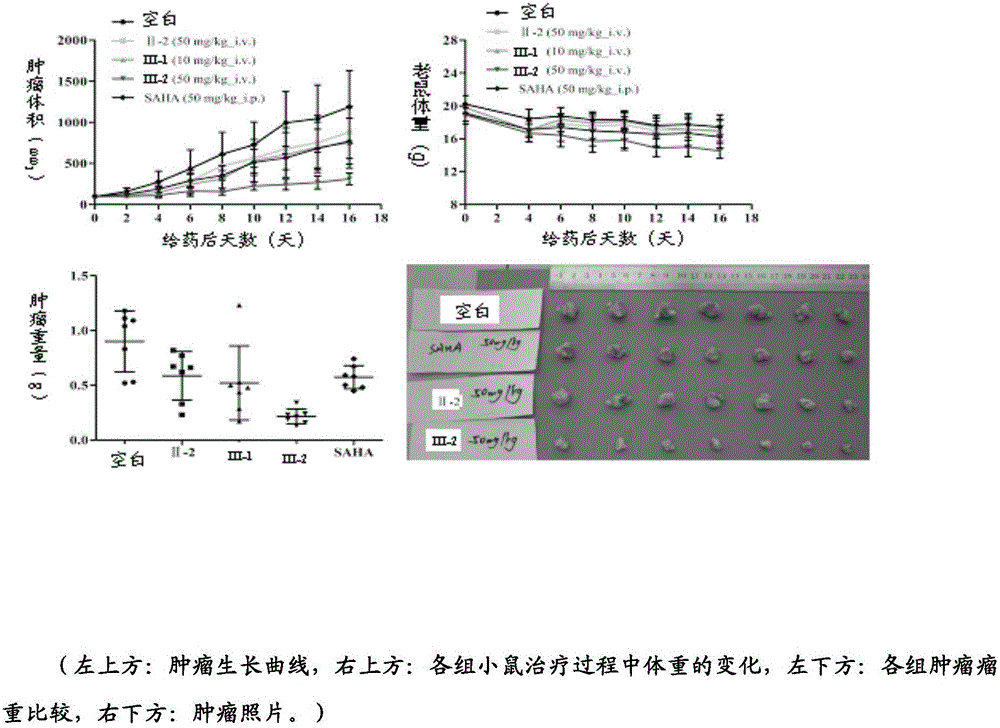
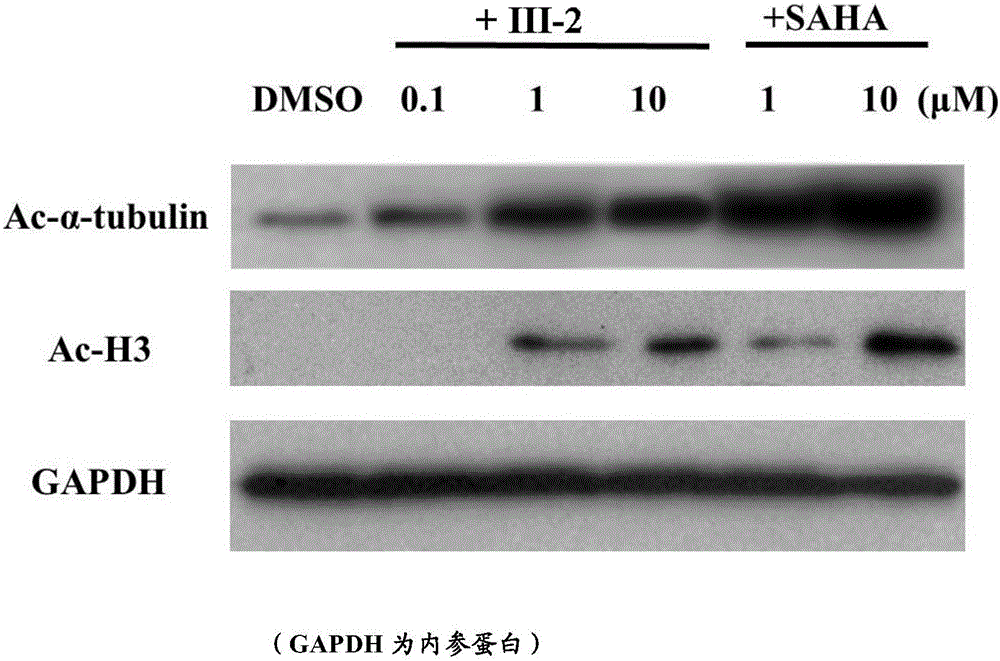
Histone deacetylase inhibitor, and preparation method and application thereof
A compound and sulfonyl technology, applied in the field of preparation of histone deacetylase inhibitor drugs, can solve the problems of large differences in drug exposure, cardiotoxicity, poor tolerance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0333] Synthesis of Compound 1-(Methoxymethoxy)-4-nitrobenzene (F1)
[0334]
[0335] Add 100mmol of p-nitrophenol to 300ml of dichloromethane, ice-water bath, and stir. Then DIPEA120mmol and MOMC1200mmol were added dropwise, and stirred at room temperature after completion. After reacting for 3 hours, the reaction solution was washed with saturated brine, water, 1N hydrochloric acid and saturated brine in sequence, and the organic layer was spin-dried under reduced pressure to obtain the dark red oily target product.
Embodiment 2
[0337] Synthesis of Compound 4-(Methoxymethoxy)aniline (F2)
[0338]
[0339] Add compound F1100mmol, 10% Pd / C5mmol to 250ml methanol, pass through hydrogen, react overnight at room temperature, after the reaction is complete, filter with diatomaceous earth, wash with ethyl acetate, combine organic layers, anhydrous NaSO 4 Drying and spin-drying under reduced pressure gave the dark red oily target product.
Embodiment 3
[0341] Synthesis of Compound 4-(Methoxymethoxy)-N-Methylaniline (F3)
[0342]
[0343] 100mmol Na was added to 200ml methanol several times in batches, and after the reaction was complete, 20mmol compound F2 and 28mmol paraformaldehyde were added into the reaction flask, and reacted overnight at room temperature. Then add 20mmol NaBH4 in batches, and heat to reflux for 2h. The reaction solution was concentrated under reduced pressure, the residue was added to 2N NaOH solution, extracted with tert-butyl methyl ether, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the target product in the form of dark red oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com