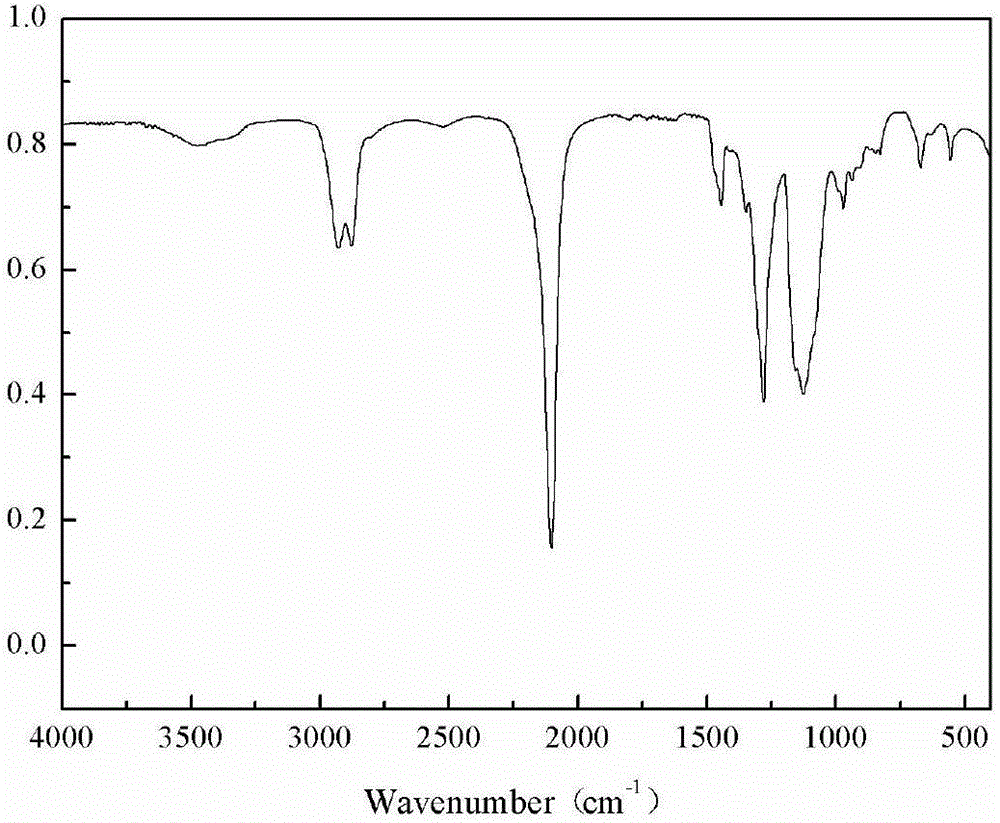
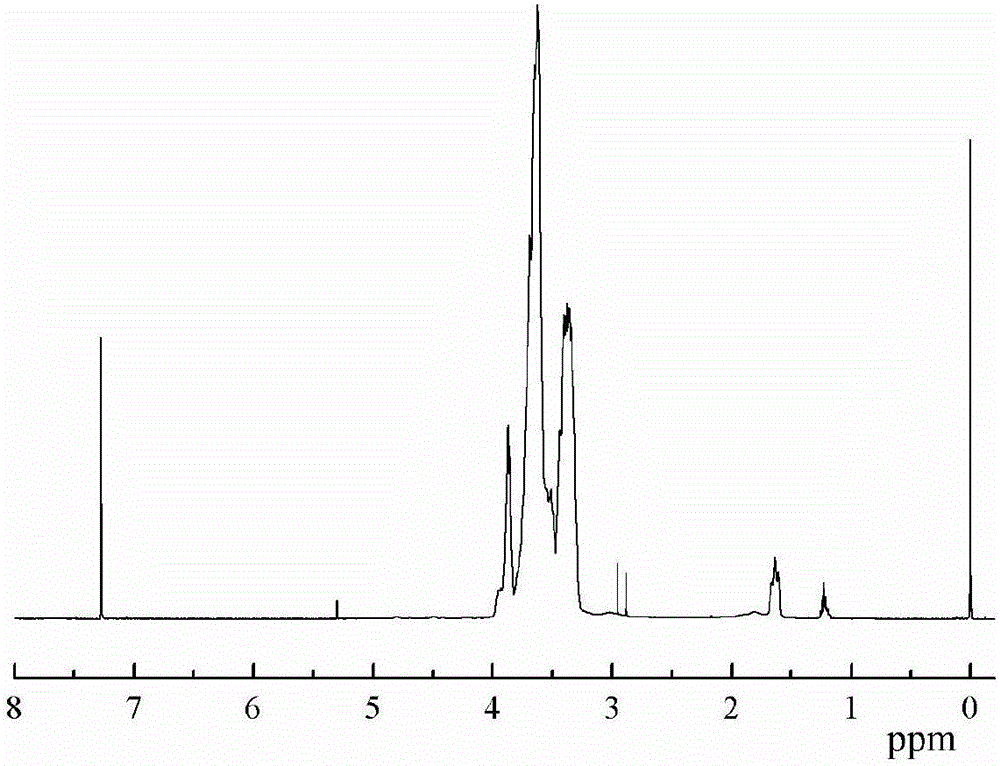
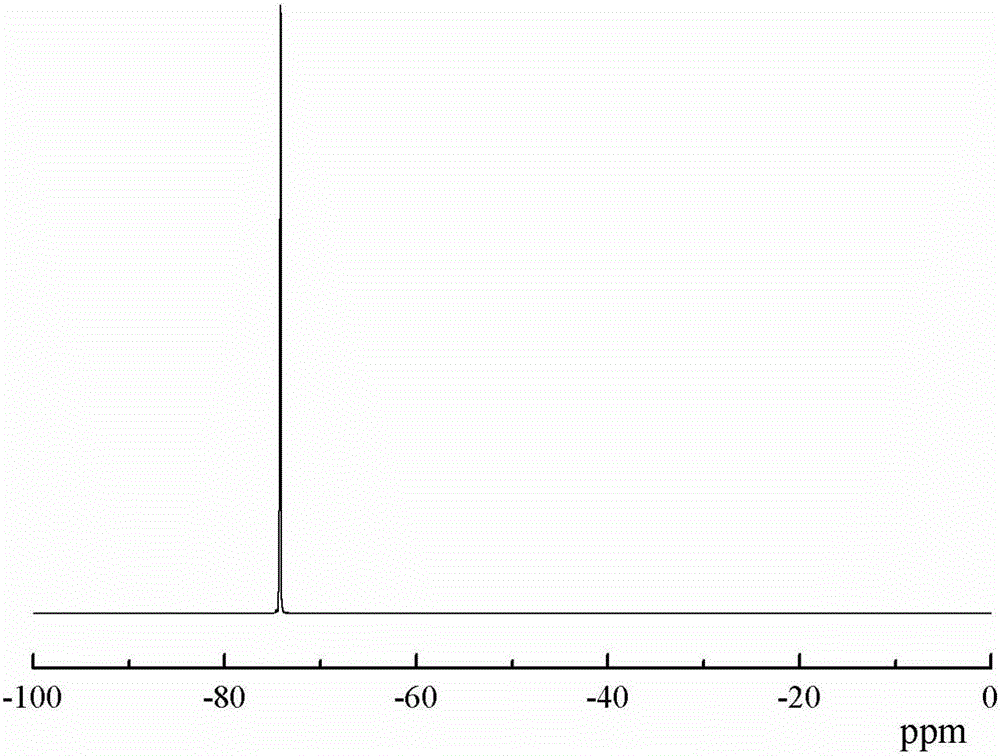
Trifluoromethyl substituted azide polymer and preparation method thereof
A technology of trifluoromethyl and polymer, which is applied in the field of trifluoromethyl-substituted azide polymer and its preparation, trifluoromethyl-substituted polyazide glycidyl ether and its preparation field, which can solve the viscosity of GAP adhesive Large hygroscopicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh and add epichlorohydrin (138.8g, 1.5mol), trifluoroethanol (30g, 0.3mol) and NaOH (18g, 0.45mol) successively in the reaction flask equipped with mechanical stirring rod, reflux condenser and thermometer, stir To complete dissolution, and rapidly stirred at 80 ℃ for 12h. After the reaction is completed, the organic phase is separated, washed and dried, and rectified by a distillation tower to obtain trifluoroethanol methyl ether-based oxirane.
[0037] Add butanediol (0.9g, 10mmol), boron trifluoride diethyl ether (0.43g, 3mmol) and 20mL dichloromethane sequentially into a reaction flask equipped with mechanical stirring, a thermometer and a reflux device, stir at 25°C for 30min, and use A constant pressure titration funnel was added dropwise a mixed monomer of epichlorohydrin (22.2 g, 0.24 mol) and trifluoroethanol methyl ether oxirane (9.36 g, 0.06 mol) to form a reaction system. The reaction system was stirred and reacted at 25° C. for 24 h. After the reaction...
Embodiment 2
[0052] Weigh and add epichlorohydrin (27.6g, 0.3mol), trifluoroethanol (30g, 0.3mmol) and NaOH (24g, 0.6mol) successively in the reaction flask equipped with mechanical stirring rod, reflux condenser and thermometer, stir To completely dissolve, and react rapidly at 100°C for 72h. After the reaction, the organic phase was separated, washed and dried, and rectified by a distillation tower to obtain trifluoroethanol methyl ether oxirane.
[0053] Add butanediol (0.9g, 10mmol), boron trifluoride diethyl ether (0.29g, 2mmol) and 60mL dichloromethane successively into the reaction flask equipped with mechanical stirring, thermometer and reflux device, stir at 25°C for 30min, use A constant pressure titration funnel was added dropwise a mixed monomer of epichlorohydrin (46.6 g, 0.5 mol) and trifluoroethanol methyl ether oxirane (15.6 g, 0.1 mol) to form a reaction system. The reaction system was stirred and reacted at -10°C for 72 hours. After the reaction was completed, 60 mL of a...
Embodiment 3
[0056] Add epichlorohydrin (276g, 3mol), trifluoroethanol (30g, 0.3mol) and NaOH (12g, 0.3mol) and NaOH (12g, 0.3mol) successively in the reaction bottle equipped with mechanical stirring rod, reflux condenser and thermometer, stir until completely Dissolved and reacted with rapid stirring at 60°C for 6h. After the reaction is completed, the organic phase is separated, washed and dried, and rectified by a distillation tower to obtain trifluoroethanol methyl ether-based oxirane.
[0057] Add butanediol (0.9g, 10mmol), boron trifluoride diethyl ether (1.15g, 8mmol) and 20mL dichloromethane sequentially into a reaction flask equipped with mechanical stirring, a thermometer and a reflux device, stir at 25°C for 30min, and use A constant pressure titration funnel was added dropwise a mixed monomer of epichlorohydrin (1.85 g, 0.02 mol) and trifluoroethanol methyl ether oxirane (15.6 g, 0.1 mol) to form a reaction system. The reaction system was stirred and reacted at 30° C. for 72 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com