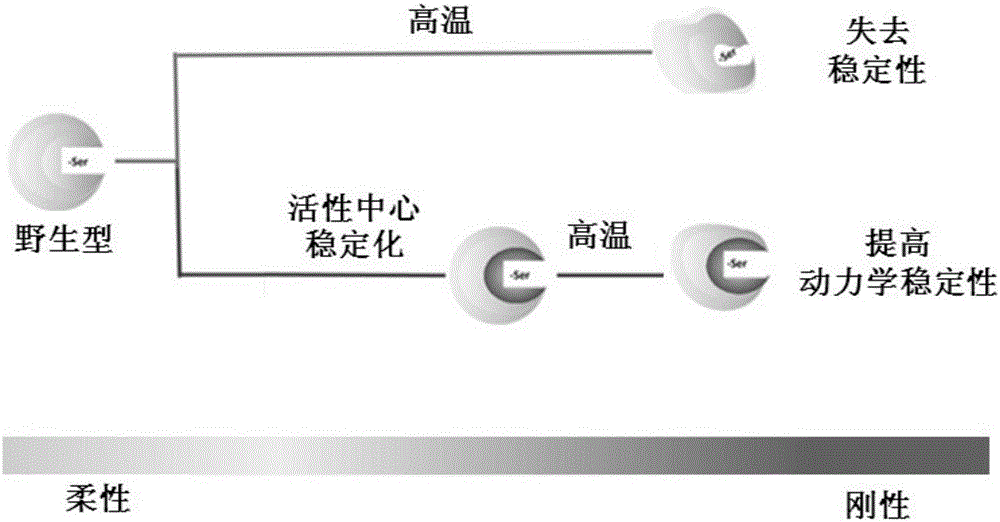
Universal strategy for efficiently improving enzyme thermodynamics stability
A thermodynamic and stable technology, applied in the direction of enzymes, hydrolases, biochemical equipment and methods, etc., can solve the problems of loss of catalytic reaction function, easy inactivation and denaturation of LIP1, weak stability restricting application, etc., and achieve half-life improvement. , the effect of improving stability and optimum temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the selection of mutation site
[0040] The wild-type (WT) amino acid sequence of Candida rugosa lipase 1 involved in the present invention is shown in SEQ ID NO: 1, and its nucleotide sequence is shown in SEQ ID NO: 9.
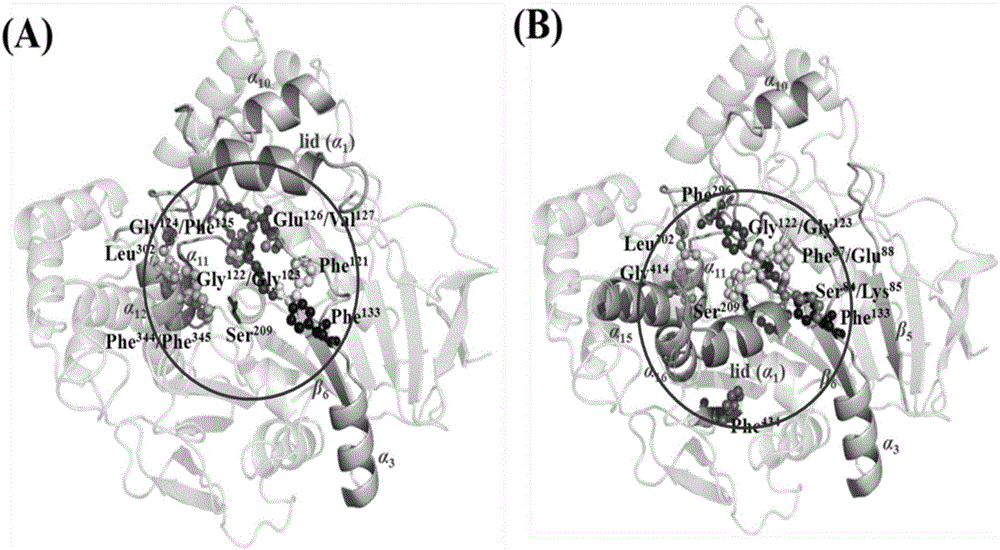
[0041] The crystal structure of LIP1 was searched in the PDB database, and the structure with the highest resolution was selected as the research object. Since LIP1 has a helical segment called "lid" above the active pocket, resulting in two structures of LIP1 in solution: Open and Closed, the two structures were analyzed simultaneously when selecting the target site. Both are centered on the catalytic residue Ser209, and the surrounding All amino acids within (use PyMOL command: PyMOL->select AA, polymer within 10 of Ser209 to find). Then use the B-FITTER software to analyze the B-factor values of these amino acids, arrange them in order of value from large to small, and select the top 11 amino acids with the largest B-factor in eac...
Embodiment 2
[0045] Example 2, site-directed saturation mutation construction gene mutation library
[0046] The primers for constructing the saturation mutation library are shown in Table 2.
[0047] Table 2 is used to construct the primer of saturation mutant library
[0048]
[0049]
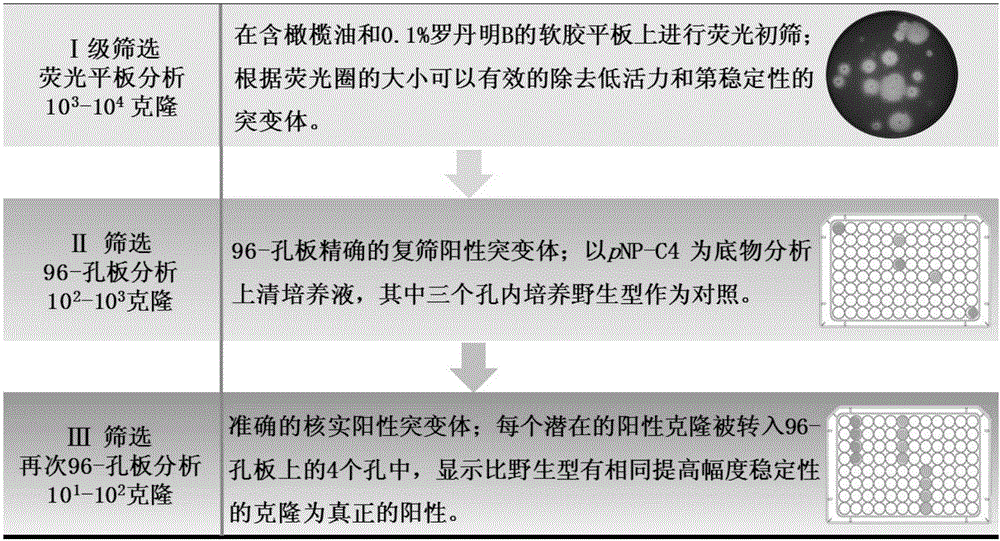
[0050] Under the action of PrimerSTAR max DNA polymerase (TaKaRa Company), the whole plasmid was amplified using the wild-type gene of LIP1 as a template. PCR reaction conditions: pre-denaturation at 98°C for 5 min, each cycle of denaturation at 98°C for 10 s, annealing at 55°C for 5 s, extension at 72°C for 2.5 min, a total of 30 cycles; the final extension at 72°C for 5 min. After the PCR product was purified, it was directly transformed into Escherichia coli DH5α competent, heat-shocked at 42°C for 90s, and incubated at 37°C for 1h. Then spread it on the LB plate containing 25μg / ml Zeocin resistance. After the clones grow out, directly wash all the clones from the plate with sterile water, coll...
Embodiment 3
[0051] Embodiment 3, the preparation of Pichia pastoris competent cell
[0052] Pick a single colony of Pichia pastoris from the YPD plate, inoculate it in 4ml of YPD liquid medium, and cultivate it with shaking at 30°C overnight for about 24-48 hours, and the OD is about 6-8. Dispense the culture solution into 1.5ml EP tubes, centrifuge at 4°C, 4000rpm for 5min, and discard the supernatant. The precipitate was washed with ice-cold sterile water, centrifuged at 4°C and 4000 rpm for 5 min, and the supernatant was discarded. The precipitate was treated with 1ml of lithium acetate solution, and after the precipitate was resuspended, it was placed at 30°C for 30min. Then centrifuge at 4°C, 4000rpm for 5min, and discard the supernatant. (Lithium acetate solution: 10mM pH7.5 Tris-HCL; 10mM DTT; 100mM LiAC) The precipitate was washed with ice-cold sterile water, centrifuged at 4000rpm for 5min at 4°C, and the supernatant was discarded. The precipitate was washed with ice-cold 1M D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com