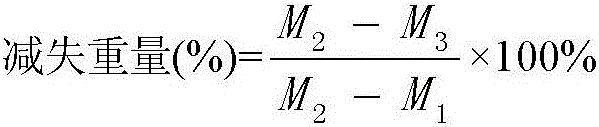Water-soluble vitamin composition for injection and quality control method thereof
A technology for water-soluble vitamins and injections, which can be used in drug combinations, active ingredients of heterocyclic compounds, drug delivery, etc., and can solve problems such as insufficient stability, insufficient synthesis amount, and vitamin deficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
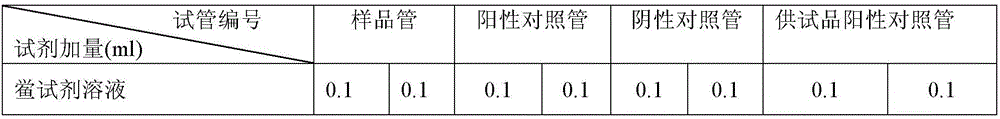
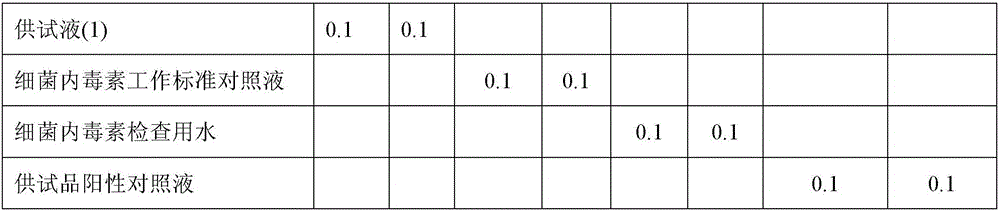
Examples
Embodiment 1
[0219] Embodiment 1: Preparation of water-soluble vitamin composition for injection
[0220] Weight ratio per bottle: thiamine nitrate 3.1mg, nicotinamide 40mg, pyridoxine hydrochloride 4.9mg, sodium pantothenate 16.5mg, riboflavin sodium phosphate 4.9mg, vitamin C sodium 113mg, biotin 60ug, folic acid 0.4mg, 5.0 ug vitamin B 12 , Methylparaben 0.5mg, Glycine 300mg, Disodium edetate 0.5mg.
[0221] Preparation method:
[0222] (1) Weigh 12 kinds of materials, add water for injection (the amount is 800 times the weight of thiamine nitrate), stir evenly, adjust the pH value to 5.7-6.0 with 1mol / L sodium hydroxide solution or hydrochloric acid solution, raw and auxiliary materials Dissolve completely, add water for injection to 1000 times the weight of thiamine nitrate, add activated carbon at 0.1w / v% by volume of the drug solution for adsorption for 30min, filter for decarburization, and adjust the pH value of the solution to 5.7-6.0 with an acid-base regulator;
[0223] (2) ...
Embodiment 2
[0225] Embodiment 2: Preparation of water-soluble vitamin composition for injection
[0226] Weight ratio per bottle: thiamine nitrate 3.4mg, nicotinamide 39mg, pyridoxine hydrochloride 5.3mg, sodium pantothenate 15.5mg, riboflavin sodium phosphate 5.3mg, vitamin C sodium 110mg, biotin 65ug, folic acid 0.38mg, 5.9 ug vitamin B 12 , Methylparaben 0.45mg, Glycine 325mg, Disodium edetate 0.4mg.
[0227] Preparation method: Carry out as in Example 1, but use freeze-drying curve B to carry out freeze-drying.
Embodiment 3
[0228] Embodiment 3: Preparation of water-soluble vitamin composition for injection
[0229] Weight ratio per bottle: thiamine nitrate 2.9mg, nicotinamide 43mg, pyridoxine hydrochloride 4.7mg, sodium pantothenate 17.5mg, riboflavin sodium phosphate 4.8mg, vitamin C sodium 123mg, biotin 58ug, folic acid 0.43mg, 5.0 ug vitamin B 12 , Methylparaben 0.60mg, Glycine 275mg, Disodium edetate 0.6mg.
[0230] Preparation method: carry out according to embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com