Hydrophilic domain protein gene
A gene and protein protection technology, which is applied in the application field of protecting enzyme activity and reducing enzyme aggregation, can solve the problems of unknown and unclear anti-stress protection mechanism of hydrophilic proteins, and achieve the effect of preventing structural changes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Analysis of Hydrophilic Domain of Dhp Protein HD
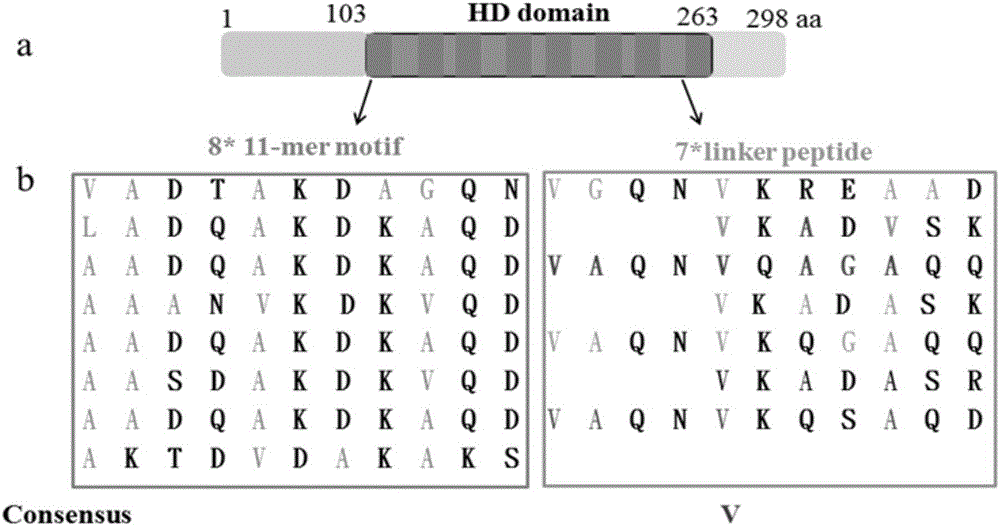
[0044] The remarkable feature of the Dhp protein is that it contains a hydrophilic domain (HD), which has a hydrophilic amino acid content of 63.9%. The HD domain contains 8 motifs consisting of 11 amino acid residues. Between the two motifs There are connecting peptides between them, and there are 7 connecting peptides in total, 4 of which are composed of 11 amino acid residues and 3 of which are composed of 7 amino acid residues. Such as figure 1 As shown, the motif analysis of 11 amino acid residues found that the amino acids at the 3rd, 4th, 6th, 7th, 8th, 10th and 11th positions are hydrophilic amino acids (hydrophilic amino acid content is 61.36%) , the amino acids at other positions are hydrophobic amino acids. The motif sequence is characterized by [ΦΦE / QXΦKE / QKΦXE / D / Q], where Φ is a hydrophobic amino acid. For the connecting peptide analysis of 11 amino acids, it was found (55.38% of the hydrophili...
Embodiment 2
[0045] Example 2 Construction of HD hydrophilic domain protein expression vector
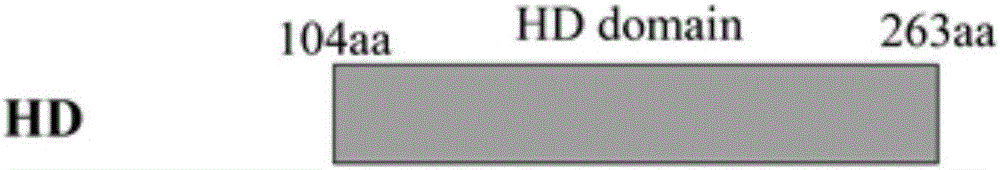
[0046] In order to study the protective function of the HD hydrophilic domain, this study analyzed the HD domain, recombined the domains and constructed expression vectors respectively. According to the positions of the 8 motifs composed of 11 amino acids in the Dhp protein, we Divide the Dhp protein into image 3Structures shown: core protein HD (amino acid residue sequence 104-263), and recombinant protein 2HD (containing twice the core protein HD amino acid sequence).
[0047] Using the recombinant plasmid pET28a-dhp stored in our laboratory as a template, add PCR-specific primers to amplify the complete nucleotide sequence: PCR reaction conditions: 98°C for 5min, [98°C for 30sec, 64°C for 30sec, 72°C for 1min] After 33 cycles, 72°C for 10 min, the PCR products were gel-recovered. The DNA product and pET28a vector were digested with EcoRI / NdeI and ligated overnight at 4°C or 16°C. Take 10 ...
Embodiment 3
[0048] Example 3 Induced expression and purification of HD hydrophilic domain protein in Escherichia coli
[0049] (1) Experimental method
[0050] 1. IPTG induces HD hydrophilic domain protein expression
[0051] 1) Streak activation of the successfully constructed recombinant plasmids BL-hd and BL-2hd strains. Pick a single colony and place it in 3 mL of fresh LB medium (containing kanamycin 50 mg / mL), and cultivate overnight at 37° C. and 220 rpm with shaking.
[0052] 2) The next day, the seed solution was transferred to 20mL LB medium containing Km (50mg / mL) at a ratio of 1:100, and cultured with shaking at 37°C and 220rmp, OD 600 About 0.6-0.8.
[0053] 3) Adding IPTG with final concentrations of 0.5, 1.0, and 1.5 mM respectively at 16° C. overnight (without adding IPTG as a blank control) to induce Escherichia coli to perform exogenous expression.
[0054] 4) Take 2 mL of the induced bacterial solution, centrifuge at 5,000 rpm for 5 minutes, and collect the bacteria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com