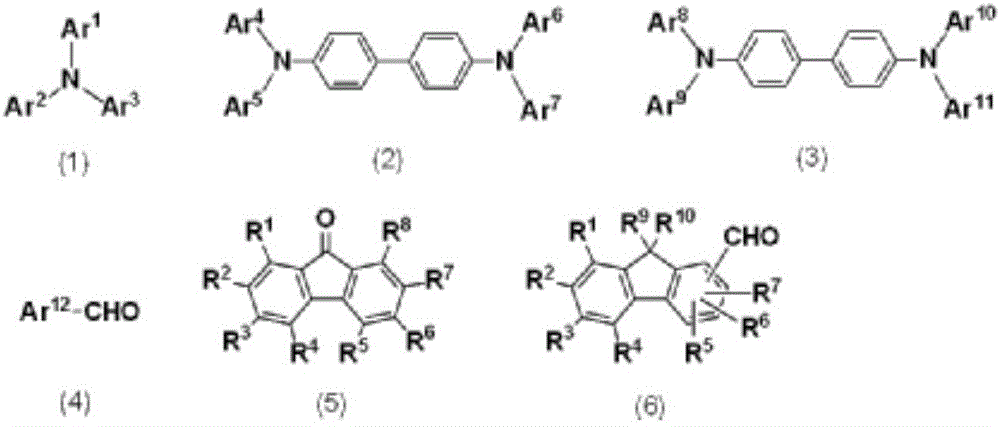
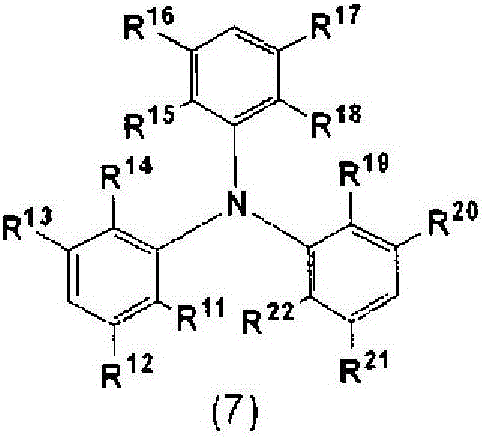
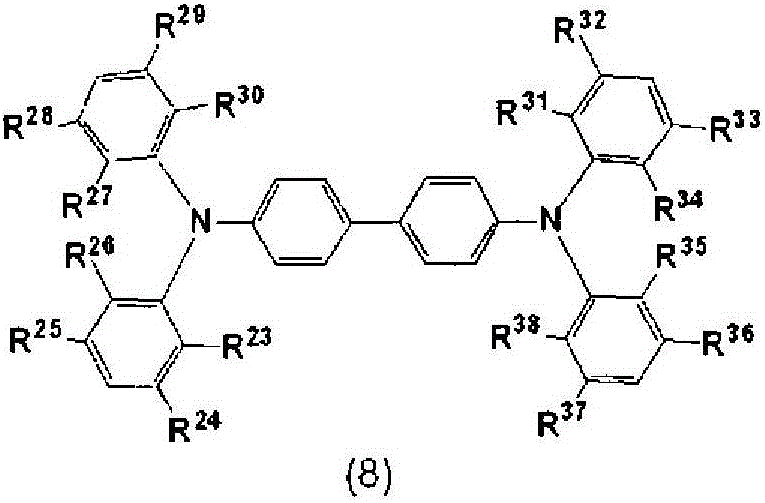
Charge-transporting varnish
A technology of charge transport and varnish, applied in the direction of circuits, electric light sources, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0253] Hereinafter, the present invention will be described more specifically with reference to synthesis examples, examples, and comparative examples, but the present invention is not limited to the following examples. In addition, the apparatuses used are as follows.
[0254] (1) NMR: ECX-300 manufactured by JEOL Ltd.
[0255] (2) LC / MS: ZQ2000 manufactured by Waters Co., Ltd.
[0256] (3) Substrate cleaning: Substrate cleaning device manufactured by Choshu Sangyo Co., Ltd. (decompression plasma method)
[0257] (4) Coating of varnish: spin coater MS-A100 manufactured by Mikasa Co., Ltd.
[0258] (5) Film thickness measurement: Micro shape measuring machine Surfcorder ET-4000 manufactured by Kosaka Laboratory
[0259] (6) Determination of polymer molecular weight: manufactured by Shimadzu Corporation (column: SHODEX GPC KF-803L+GPC KF-804L, column temperature: 40°C, detector: UV detector (254nm) and RI detector, Eluent: THF, column flow rate: 1.0ml / min.)
[0260] (7) Ma...
Synthetic example 1
[0263] [Synthesis Example 1] Synthesis of Compound 1
[0264] [chem 29]
[0265]
[0266] Add potassium carbonate (7.60g, 55mmol) and diethylene glycol 2-bromoethyl methyl ether (9535μL, 55mmol) to 3-bromophenol (8.65g, 50mmol) in acetonitrile solution (170mL), stir at room temperature for 18 After 1 hour, heat to reflux for 7 hours. Potassium carbonate (1.50 g, 10 mmol) and diethylene glycol 2-bromoethyl methyl ether (1900 μL, 10 mmol) were added to the suspension, followed by heating under reflux for 4 hours. After the reaction, the insoluble matter was removed by filtration, the filtrate was concentrated, and the obtained crude product was purified by silica gel column chromatography (eluent: hexane / ethyl acetate) to obtain compound 1 (16.13 g, 100% yield) as a colorless liquid. Rate).
[0267] 1 H-NMR (300MHz, CDCl 3 ): δ3.38(s, 3H), 3.53-3.58(m, 2H), 3.64-3.75(m, 6H), 3.84(app t, J=4.8Hz, 2H), 4.11(app t, J=4.8 Hz, 2H), 6.83-6.87(m, 1H), 7.05-7.16(m, 3H).
[026...
Synthetic example 2
[0269] [Synthesis Example 2] Synthesis of Compound 2
[0270] [chem 30]
[0271]
[0272] To N,N'-diphenylbenzidine (4.88g, 14.5mmol), compound 1 (11.15g, 35mmol) prepared in Synthesis Example 1 in toluene suspension (70mL), add Pd(dba) 2 (834mg, 1.5mmol), t-BuONa (4.18g, 43.5mmol), [(t-Bu) 3 PH]BF 4 (841mg, 2.9mmol), toluene (30mL), after nitrogen replacement, heated to reflux for 3 hours. After the reaction was completed, it was filtered with celite, the filtrate was concentrated, and the obtained crude product was purified by silica gel column chromatography (eluate: hexane / ethyl acetate) to obtain Compound 2 (12.06 g, 100% yield) as a brown liquid. Rate).
[0273] 1 H-NMR (300MHz, CDCl 3 ): δ3.36(s, 6H), 3.52-3.55(m, 4H), 3.62-3.73(m, 12H), 3.80(app t, J=4.8Hz, 4H), 4.04(app t, J=4.8 Hz, 4H), 6.57-6.59(m, 2H), 6.68-6.71(m, 4H), 7.02(app t, J=7.2Hz, 2H), 7.11-7.17(m, 10H), 7.23-7.28(m , 4H), 7.44 (d, J=8.7Hz, 4H).
[0274] LC / MS (ESI + )m / z; 813[M+1] +
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com