Application of frankincense pentacyclic triterpenic acid compound or salt and combination to preparation of diabetes treating medicine
The technology of a compound and triterpene acid, which is applied in the field of medicine, can solve problems such as reports on the pharmacological effects of boswellic acid compounds on lowering blood sugar.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
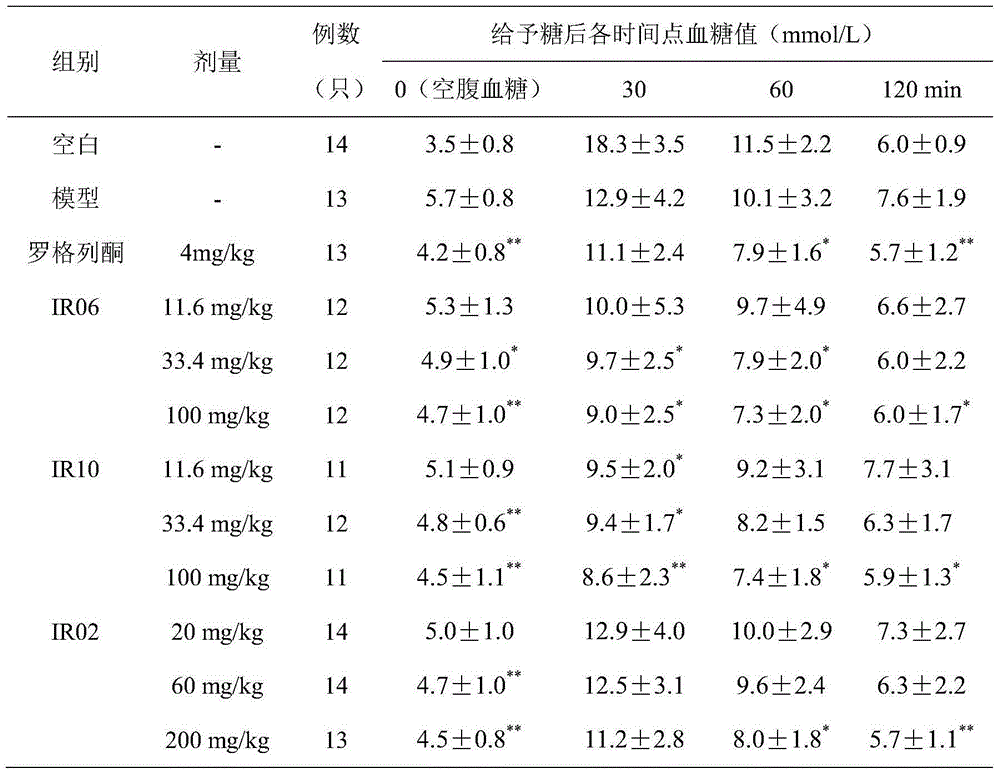
[0015] Example 1. Therapeutic effect of boswellic acid compounds on dexamethasone-induced insulin resistance in mice
[0016] Experimental Materials:
[0017] Animal: Kunming mouse, male.
[0018] Test substances: IR06 (AKBA), content ≥98% (HPLC), IR10 (α, β-ABA 1:1 mixture), IR02 (boswellic acid extract, containing AKBA26.4%, α-ABA10.1%, β-ABA 15.7%), prepared by Innovation Center of Tianjin Pharmaceutical Research Institute,
[0019] Positive drug: Rosiglitazone, 4mg / tablet, batch number: 121001, Chengdu Hengrui Pharmaceutical Co., Ltd.
[0020] Instrument: blood glucose meter, model, FreeStyle Freedom, product of Abbott Diabetes Care Company.
[0021] experimental method:
[0022] Model preparation: mice, male, weighing 18-21g, fasted for 16 hours, measured fasting blood sugar, excluded animals with abnormal blood sugar, intramuscularly injected dexamethasone 2mg / kg, injected once a day, continuously injected for 14 days, blank control group was injected accordingly sa...
Embodiment 2
[0028] Example 2. The therapeutic effect of boswellic acid compounds on hereditary diabetes animal model (Kkay mice)
[0029] Experimental Materials:
[0030] Animals: Kkay mice, female. Purchased from Union Institute of Zoology, Chinese Academy of Medical Sciences;
[0031] Test substances: IR06 (AKBA), content ≥98% (HPLC), IR10 (α, β-ABA 1:1 mixture), IR02 (boswellic acid extract, containing AKBA26.4%, α-ABA10.1%, β-ABA 15.7%), prepared by the Innovation Center of Tianjin Pharmaceutical Research Institute;
[0032] Positive drug: Rosiglitazone, 4mg / tablet, batch number: 121001, Chengdu Hengrui Pharmaceutical Co., Ltd.;
[0033] Instrument: blood glucose meter, model, FreeStyle Freeβdom, product of Abbott Diabetes Care.
[0034] experimental method:
[0035] Model preparation: Kkay mice were fed adaptively for 1 week and weighed 26-33g. Blood was collected from the tail vein to measure the blood glucose level randomly, and the random blood glucose level reached the stand...
Embodiment 3
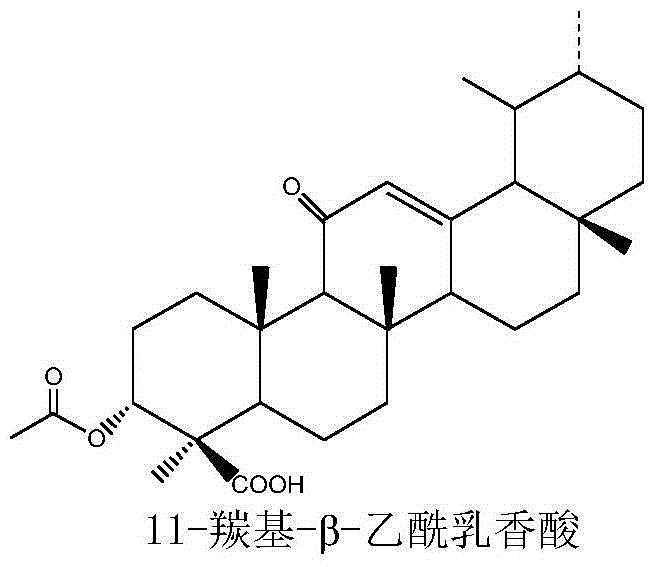
[0043] 100g of frankincense resin, add 1L of medicinal ethanol for reflux extraction for 1.5 hours each time, extract continuously for 2 times, combine the extracts, concentrate to about 300ml, add 700ml of 5% sodium carbonate solution to disperse and suspend, and use petroleum ether-ethyl acetate (3: 2) Extract 3 times, adjust the pH of the water layer to 3-4 with 10% HCl, extract 4 times with ethyl acetate, combine the ethyl acetate layers, wash with water until the water is neutral, add anhydrous Dehydrated with sodium sulfate and concentrated to dryness to obtain 32 g of boswellic acid refined extract (IR02).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com