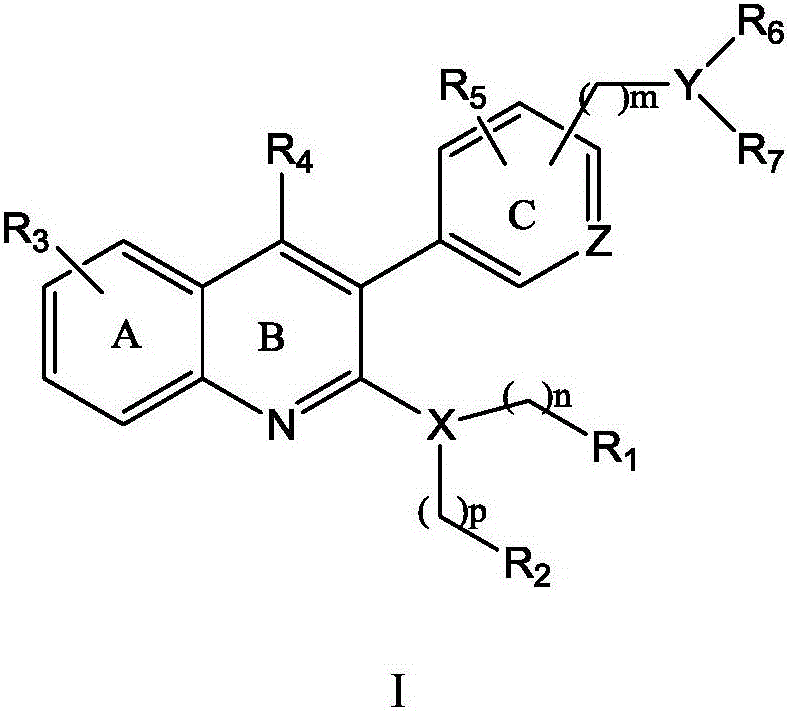
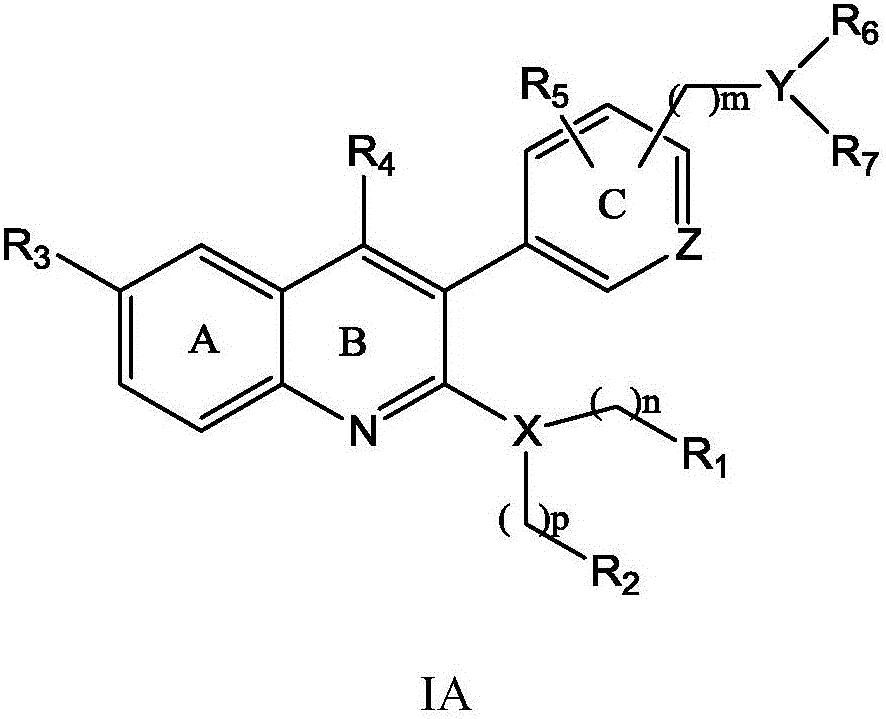
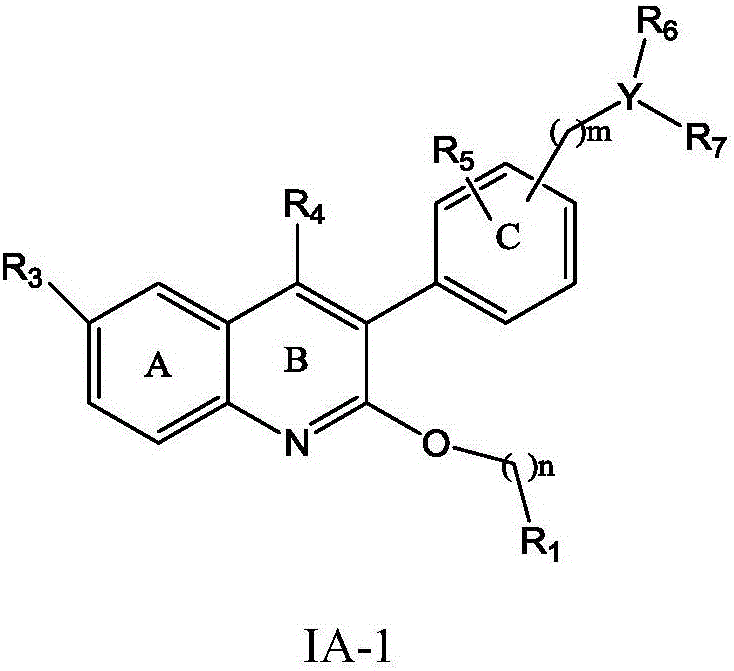
Quinoline derivative, preparation method and application thereof
A quinoline and compound technology, applied in the field of medicine, can solve the problems of increasing the hidden danger of drug use, reducing the effective blood drug concentration, increasing the adverse drug reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0318] Preparation of 3-(4-N,N-dimethylaminomethyl)phenyl-2-(1-naphthylmethoxy)-6-bromoquinoline (1)
[0319]
[0320] (1-1) Preparation of intermediate 3-iodo-6-bromoquinoline
[0321]
[0322]Dissolve 6-bromoquinoline (5.00g, 24.03mmol) in 40mL of acetic acid, add N-iodosuccinimide (5.95g, 26.44mmol) in batches, stir and react at 80°C for 20 hours, add 150mL of water , extracted three times with dichloromethane, combined the organic phases, washed with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, and column chromatography (petroleum ether / ethyl acetate 4:1) gave 3.80 g of yellow solid, yield: 47.37%. 1 H NMR (300MHz, CDCl 3 )δ9.03(d, J=2.1Hz, 1H), 8.45(d, J=1.5Hz, 1H), 7.93(d, J=9.0Hz, 1H), 7.87(d, J=2.1Hz, 1H) ,7.79(dd,J=9.0,2.1Hz,1H).m / z[M+H] + : 333.8715.
[0323] (1-2) Preparation of intermediate N-oxo-6-bromo-3-iodoquinoline
[0324]
[0325] Dissolve 6-bromo-3-iodoquinoline (3.40g, 10.21mmol) in 40mL of chloroform, add 3-chloroperoxy...
Embodiment 2
[0336] Preparation of 3-((4-N-methylpiperazine)phenyl)-2-(1-naphthylmethoxy)-6-bromoquinoline (2)
[0337]
[0338] Dissolve 2-(1-naphthylmethoxy)-6-bromo-3-iodoquinoline (100mg, 0.21mmol) in 3mL of toluene, add Pd(PPh 3 ) 4 (13mg, 0.01mmol), 1mL aqueous solution of sodium carbonate (43mg, 0.41mmol), 4-(N-methylpiperazine) phenylboronic acid (55mg, 0.25mmol), stirred at 80°C for 10 hours, added 5mL of water, Chloromethane was extracted three times, and the combined organic phases were subjected to column chromatography (dichloromethane / methanol 20:1) to obtain 89 mg of a yellow solid with a yield of 78.90%. m.p.:134-135℃.1H NMR (400MHz, Acetone-d 6 )δ8.26(d, J=8.5Hz, 1H), 8.14(s, 1H), 8.10(d, J=2.2Hz, 1H), 7.96(d, J=7.6Hz, 1H), 7.91(d, J=8.3Hz, 1H), 7.84(d, J=8.9Hz, 1H), 7.80–7.74(m, 2H), 7.63–7.59(m, 1H), 7.59–7.53(m, 3H), 7.49(dd ,J=8.2,7.1Hz,1H),6.96–6.87(m,2H),6.09(s,2H),3.24–3.15(m,4H),2.50–2.41(m,4H),2.24(s,3H ).m / z[M+H] + :538.1482.
Embodiment 3
[0340] Preparation of 3-(4-(morpholine-1-methyl)phenyl)-2-(1-naphthylmethoxy)-6-bromoquinoline (3)
[0341]
[0342] Dissolve 2-(1-naphthylmethoxy)-6-bromo-3-iodoquinoline (150mg, 0.31mmol) in 3mL of toluene, add Pd(PPh 3 ) 4 (18mg, 0.02mmol), 2mL aqueous solution of sodium carbonate (97mg, 0.92mmol), 4-(4-morpholine-1-methyl) phenylboronic acid (81mg, 0.26mmol), stirred at 80°C for 10 hours, added water 5 mL was extracted three times with dichloromethane, the organic phases were combined, and column chromatography (dichloromethane / methanol 15:1) gave 122 mg of a yellow solid with a yield of 76.15%. 1 H NMR (600MHz, Acetone-d 6 )δ8.22(d, J=8.4Hz, 1H), 8.17(s, 1H), 8.09(d, J=2.3Hz, 1H), 7.94(dd, J=8.3, 0.9Hz, 1H), 7.88( d,J=8.3Hz,1H),7.85(d,J=8.9Hz,1H),7.78(dd,J=8.8,2.3Hz,1H),7.71(d,J=6.5Hz,1H),7.61– 7.56(m,3H),7.56–7.52(m,1H),7.45(dd,J=8.2,7.0Hz,1H),7.30(d,J=8.3Hz,2H),6.06(s,2H),3.59 (t,J=4.6Hz,4H),3.45(s,2H),2.36(br.s,4H).m / z[M+H] + :539.1293.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com