Preparation method of 5-bromine-1,2,3-benzene tricarbonic acid
A technology of trimellitic acid and concentrated sulfuric acid, applied in the field of organic bromides and polycarboxylic acid aromatics, can solve the problems of complicated preparation method and long reaction time, and achieve the effects of simple reaction conditions, simple operation and good yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The raw materials used for the synthesis of 5-bromo-1,2,3-benzenetricarboxylic acid are 1,2,3-benzenetricarboxylic acid, concentrated sulfuric acid, NBS (N-bromosuccinimide), and the mass of ice is 1.26 g, 18.4g, 1.28g, 10g.
Embodiment 2
[0022] The raw materials used for the synthesis of 5-bromo-1,2,3-benzenetricarboxylic acid are 1,2,3-benzenetricarboxylic acid, concentrated sulfuric acid, NBS (N-bromosuccinimide), and the mass of ice is 12.6 g, 184g, 12.8g, 100g.
Embodiment 3
[0024] The raw materials used for the synthesis of 5-bromo-1,2,3-benzenetricarboxylic acid are 1,2,3-benzenetricarboxylic acid, concentrated sulfuric acid, NBS (N-bromosuccinimide), and the mass of ice is 25.2 g, 368g, 25.6g, 200g.
[0025] The reaction formula of above technique is as follows:
[0026]
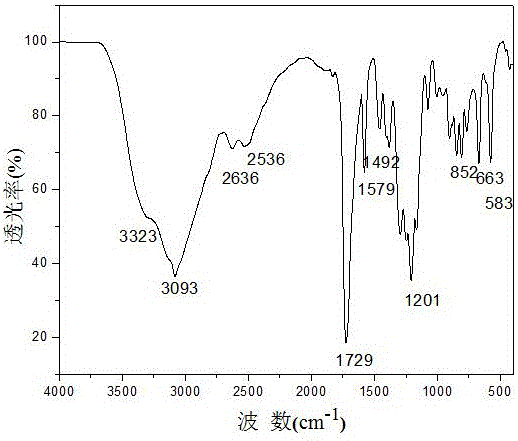
[0027] 2. Product verification
[0028] figure 1 It is the infrared spectrogram of the compound, the sample is pressed by KBr, and the measurement wavenumber range is 4000-400cm -1 . 1201cm -1 The stretching vibration of the C-O bond, 1492cm -1 、1579cm -1 The place is the vibration absorption peak of the benzene ring skeleton, 1729cm -1 The sharp peak is the stretching vibration peak of carboxyl carbonyl, 3093cm -1 is the stretching vibration peak of -OH on the carboxyl group, 3323cm -1 is the stretching vibration peak of -OH on the water molecule.
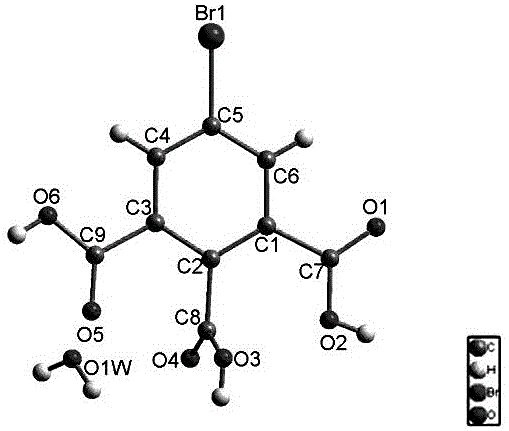
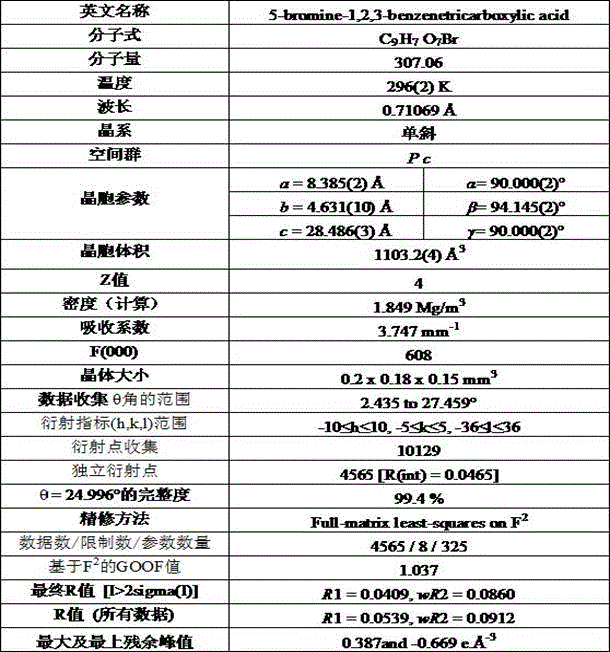
[0029] The crystal structure of the compound was determined by single crystal X-ray diffractometer. The SHELXTL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com