Similar EDTA ligand containing O-phenolic hydroxyl, as well as non-gadolinium magnetic resonance contrast agent and preparation method thereof
A technology of dihydroxyphenyl and dibenzyloxy, which is applied in the field of EDTA-like ligands containing ortho-diphenolic hydroxyl groups and non-gadolinium magnetic resonance contrast agents and their preparation, and can solve problems such as low theoretical prediction values
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 EDTA-like ligand (L) containing o-diphenolic hydroxyl group: 2,2', 2", 2"'-((3-(3,4-dihydroxyphenyl)propane-1,2 diyl ) Preparation of diaminetetraacetic acid
[0068] The chemical reaction formula is:
[0069]
[0070] According to the above chemical reaction formula, the preparation method adopted in this embodiment is as follows:
[0071] (1) Preparation of compound 2, [(N-tert-butoxycarbonyl)-amino]]-3-[(3,4-dihydroxy)-phenyl]propionic acid methyl ester:
[0072]Add thionyl chloride (15mL, 95.68mmol) into 100mL of methanol solution of L-Dopa (14.5g, 73.6mmol) under ice-bath conditions, and react at room temperature for 24 hours under stirring. After the reaction is completed, the reaction solution is depressurized The solvent methanol in the reaction liquid was distilled off to obtain the product; the obtained product was dissolved with 100 mL of saturated sodium bicarbonate and the pH of the product solution was 7 to 8, and then di-tert-butyl dicarbon...
Embodiment 2
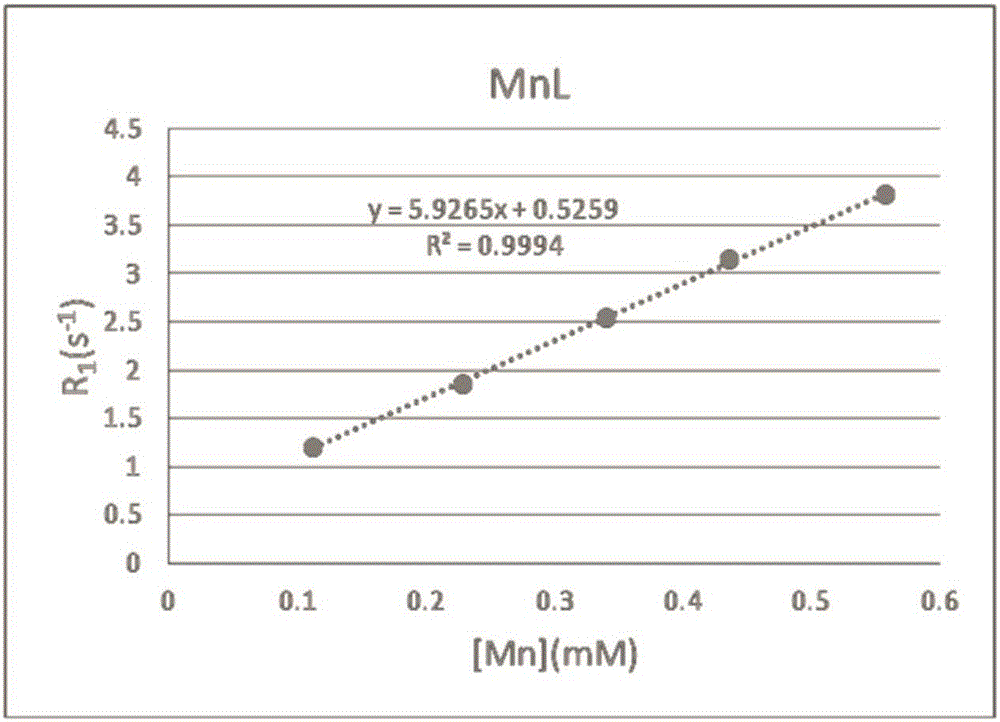
[0090] Example 2 Preparation of Paramagnetic Metal Complex Magnetic Resonance Contrast Agent (MnL)
[0091] The chemical reaction formula is:
[0092]
[0093] According to above-mentioned chemical reaction formula, the preparation method that present embodiment takes is as follows:
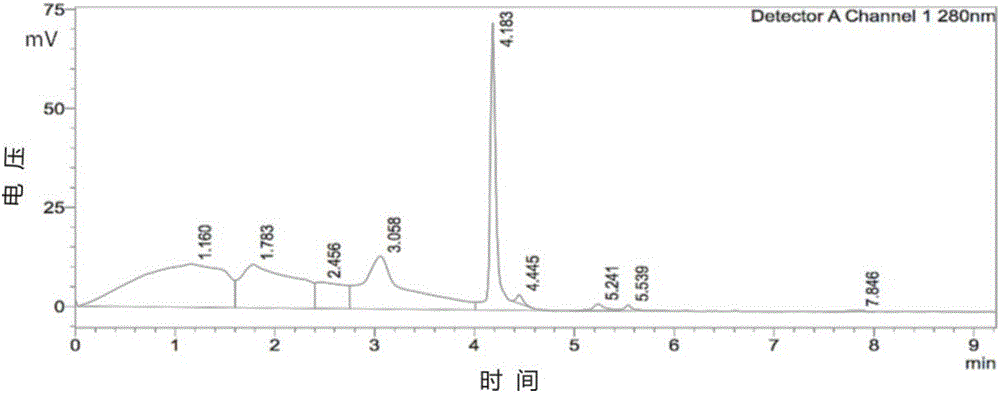
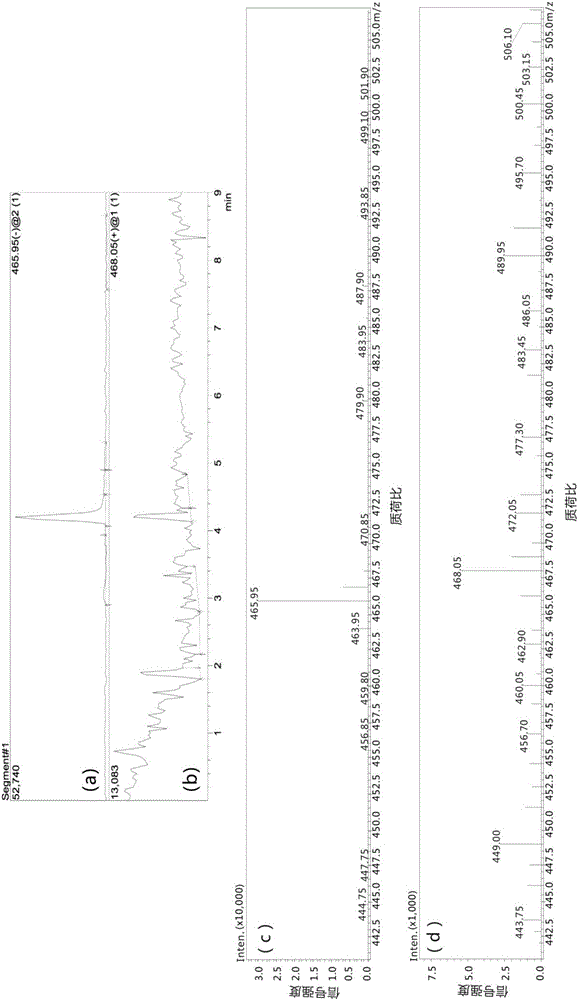
[0094] Compound 7 (0.1 g, 0.24 mmol) prepared in Example 1 was dissolved in Hepes buffer (pH=7.36, 5 mL) to obtain a compound 7 solution, and MnCl 2 4H 2 O (0.057g, 0.28mmol) was dissolved in Hepes buffer (pH=7.36, 5mL) to give MnCl 2 solution; under nitrogen protection and stirring, compound 7 solution and MnCl 2 The solution was added to the reaction vessel, stirred and reacted at room temperature under nitrogen protection for 10 minutes, and after the reaction was completed, the resulting reaction solution was filtered through a Sephdex G-25 gel column to remove Mn that did not participate in the reaction. 2+ , to obtain the Hepes solution of the Mn complex contrast agent (MnL) of the s...
Embodiment 3
[0103] Example 3 Fe coordination self-assembled metal cluster collective [Fe(MnL) 3 ] Preparation of magnetic resonance contrast agent
[0104] The chemical reaction formula is:
[0105]
[0106] According to above-mentioned chemical reaction formula, the preparation method that embodiment takes is as follows:
[0107] Compound 7 (0.1 g, 0.24 mmol) prepared in Example 1 was dissolved in Hepes buffer (pH=7.36, 5 mL) to obtain a compound 7 solution, and MnCl 2 4H 2 O (0.057g, 0.28mmol) was dissolved in Hepes buffer (pH=7.36, 5mL) to give MnCl 2 solution, the FeCl 3 (0.013g, 0.08mmol) was dissolved in Hepes buffer (pH=6.0, 2.0mL) to give FeCl 3 solution; under nitrogen protection and stirring, compound 7 solution and MnCl 2 The solution was added to the reaction vessel, and the reaction was stirred at room temperature for 10 min under the protection of nitrogen; then FeCl was added 3 Solution, under the protection of nitrogen, stirred and reacted at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com