Double-gene-deficient Salmonella enteritidis strain and establishment method thereof, and vaccine containing double-gene-deficient Salmonella enteritidis strain
A technology of Salmonella enteritidis and its construction method, applied in the direction of medical preparations containing active ingredients, bacteria, antibacterial drugs, etc., can solve the problems of systemic transmission, easy induction of wild strains, etc., to reduce fluid secretion, enhance mucosal immunity, Enhance the effect of humoral and cellular immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
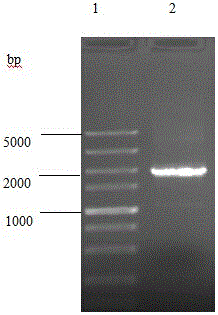
[0037] Example 1 Proliferation of Salmonella enteritidis and extraction of genome
[0038] Inoculate Salmonella enteritidis D1203 in liquid LB medium, culture overnight at 37°C, take 1.5mL of cultured bacteria, place in an Eppendorf centrifuge tube, centrifuge at 10,000g for 2min, discard the supernatant, add 1.5mL of PBS buffer to resuspend the precipitate, Centrifuge at 10,000 g for 2 minutes, discard the supernatant, add 240 μL of lysozyme to the pellet, and add 15 μL of lysozyme, ice-bath at 0°C for 30 minutes, add 15 μL of 10% SDS, mix well, add proteinase K, and bathe in water at 65°C for 30 minutes , add 200 μL of 5M NaCl solution, shake, add 500 μL of phenol / chloroform / isoamyl alcohol (volume ratio 1:24:25) solution, mix well, centrifuge at 12000 g for 10 min, take the supernatant into a centrifuge tube, record its volume, add etc. The volume of frozen absolute ethanol was placed in a -80°C refrigerator for 10 minutes, centrifuged at 12000g for 10 minutes, the supernat...
Embodiment 2
[0039] Construction of embodiment 2 rfaH gene knockout mutant strain
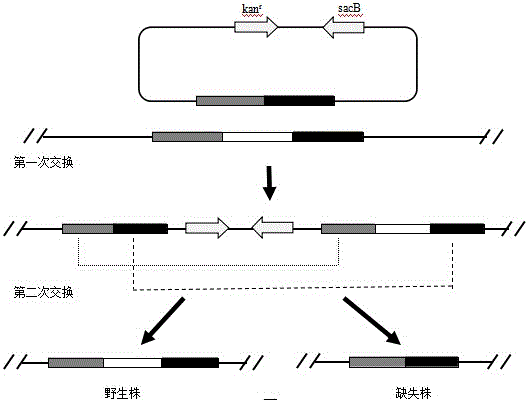
[0040] 2.1 Overlap PCR
[0041] According to the Salmonella enteritidis genome sequence published on GeneBank, the upstream primer rfaH-1F and the downstream primer rfaH-1R of the upstream homology arm sequence rU of the rfaH gene were designed, and the upstream primer rfaH- of the downstream homology arm sequence rD of the rfaH gene was designed. 2F and downstream rfaH-2R, the nucleotide sequence of the rfaH-1F is shown in SEQ ID NO.3, the nucleotide sequence of the rfaH-1R is shown in SEQ ID NO.4, the rfaH-2F The nucleotide sequence of rfaH-2F is shown in SEQ ID NO.5, and the nucleotide sequence of rfaH-2F is shown in SEQ ID NO.6. Using the above-mentioned extracted Salmonella enteritidis D1203 genomic DNA as a template, the upper and lower homology arm sequences rU and rD of rfaH were amplified by PCR method. The 50 μL PCR amplification system of rU and rD is shown in Table 1:
[0042] Table 1 PCR amp...
Embodiment 3
[0060] Example 3 Construction of Salmonella Enteritidis ΔrfaHΔsopB Double Deletion Strain
[0061] 3.1 Overlap PCR
[0062] According to the Salmonella enteritidis genome sequence published on GeneBank, the upstream primer sopB-1F and the downstream primer sopB-1R of the upstream homology arm sequence sU of the sopB gene were designed, and the upstream primer sopB- of the downstream homology arm sequence sD of the sopB gene was designed. 2F and downstream primer sopB-2R, the nucleotide sequence of the sopB-1F is shown in SEQ ID NO.9, the nucleotide sequence of the sopB-1R is shown in SEQ ID NO.10, the sopB- The nucleotide sequence of 2F is shown in SEQ ID NO.11, and the nucleotide sequence of sopB-2R is shown in SEQ ID NO.12. Using Salmonella enteritidis D1203 genomic DNA as a template, the upper and lower homology arm sequences sU and sD of sopB were amplified by PCR. The 50 μL PCR amplification system and PCR reaction conditions of sU and sD are the same as those of rU and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com