Method for inducing adipose tissue-derived stromal cells into liver sample cells
A kind of stem cell and cell induction technology, applied in the field of artificial liver, can solve the problem of long induction time, and achieve the effect of short induction time, shortened induction time and good induction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
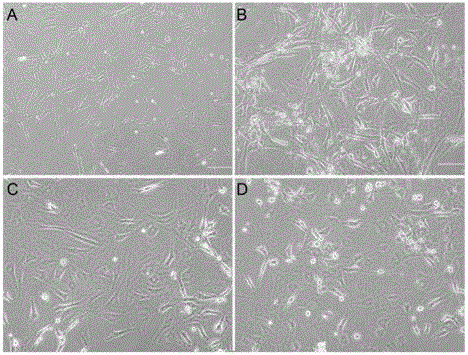
Image
Examples
Embodiment 1
[0048] 1. Isolation, extraction and primary culture of mouse ADSCs:
[0049] (1) 5 BALB / c male 3-day-old suckling mice were killed by cervical dislocation, soaked in 75% alcohol for disinfection for 3 minutes, and then transferred to an ultra-clean table;
[0050] (2) In an ultra-clean bench, use sterilized ophthalmic scissors and ophthalmic tweezers to peel off the mouse skin from below the umbilical cord, expose the inguinal adipose tissue, completely separate the inguinal adipose tissue, add 5v / v% double-antibody PBS and wash for 3 minutes, Transfer to serum-free medium for treatment;
[0051] (3) Remove the subcutaneous and fascial tissue around the mouse adipose tissue, and use ophthalmic scissors to cut it into pieces less than 1cm 3 , transferred into a 15mL centrifuge tube, added three times the volume of PBS containing 1mg / mL collagenase I, and shaken at room temperature for 1h;
[0052] (4) Equal volume of serum-containing medium to stop digestion, incubate at room...
Embodiment 2
[0065] 1. Isolation, extraction and primary culture of mouse ADSCs:
[0066] Concrete steps are with this step in embodiment 1;
[0067] 2. Subculture and cell culture;
[0068] After 72 hours of inoculation of the primary isolated cells, observe the growth of the cells, and when the cell density reaches 91%, passage at a ratio of 1:3;
[0069] Then inoculate after subculture, take the logarithmic growth of the 4th passage cells, and inoculate 1×10 4 / cm 2 After subculture, inoculate and place in 5v / v% CO 2 , Cultivated in a constant temperature incubator at 37°C; the medium is a medium containing a-MEM+20v / v%FBS;
[0070] 3. Induction culture of hepatoblast-like cells;
[0071] After 24 hours after inoculation to observe cell adhesion, the medium was replaced with hepatic induction medium, and the hepatic induction medium was changed every 4 days; the hepatic induction medium contained 50 ng / mL FGF4, 150 ng / mL HGF, 40 ng The DMEM medium containing 10v / v% FBS of / mL OSM,...
Embodiment 3
[0076] 1. Isolation, extraction and primary culture of mouse ADSCs:
[0077] Concrete steps are with this step in embodiment 1;
[0078] 2. Subculture and cell culture;
[0079] After the primary isolated cells were inoculated for 72 hours, observe the growth of the cells. If the cell density does not reach 90%, change the medium at half the volume, and pass the culture at 1:2.5 after extending the culture for one day;
[0080] Then inoculate after subculture, take the logarithmic growth of the 4th passage cells, and inoculate 1×10 4 / cm 2 After subculture, inoculate and place in 5v / v% CO 2 , Cultivated in a constant temperature incubator at 37°C; the medium is a medium containing DMEM+10v / v% FBS;
[0081] 3. Hepatoblast-like cell induction culture;
[0082] After 24 hours after inoculation to observe cell adhesion, the medium was replaced with hepatogenic induction medium, and the hepatic induction medium was changed every 4 days; the hepatic induction medium contained 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com