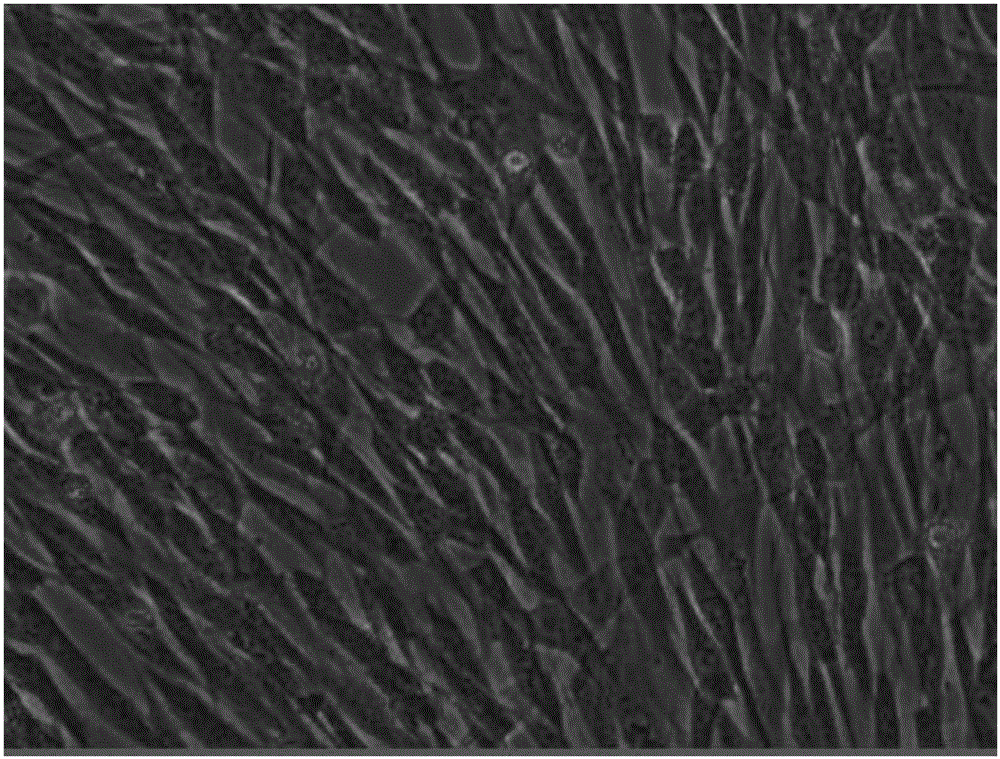
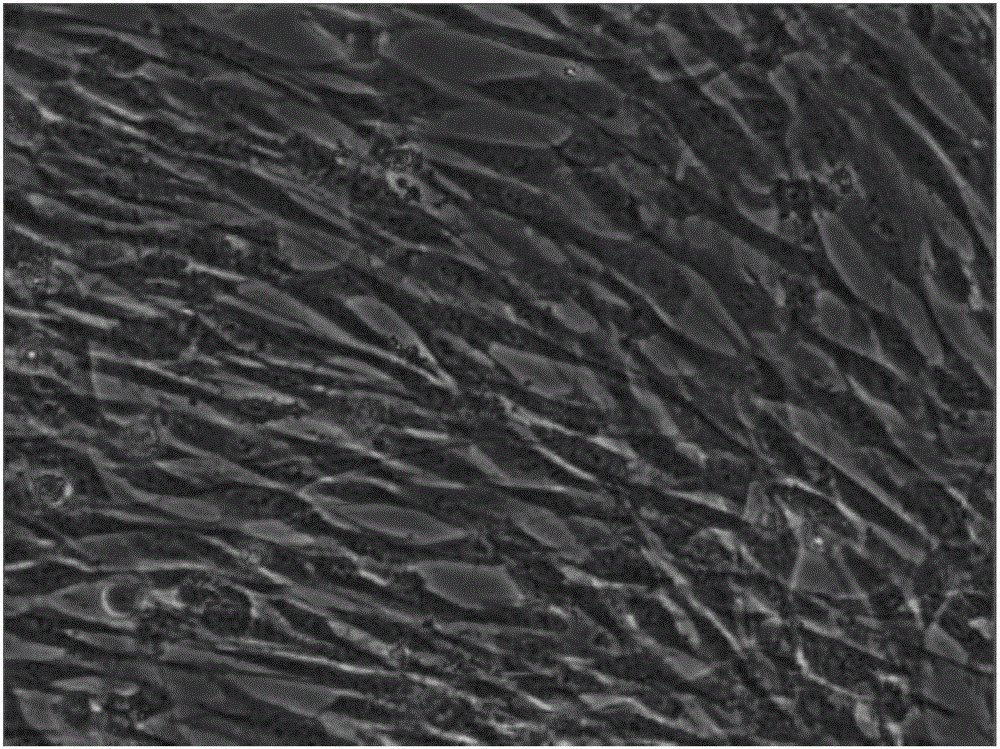
Mesenchymal stem cell serum-free culture medium
A serum-free medium and stem cell technology, applied in the field of mesenchymal stem cell serum-free medium, can solve the problem that the content of components and specific functions are not completely determined, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A serum-free medium for mesenchymal stem cells in this embodiment uses L-DMEM medium as the base medium, and also includes the following additional components, and the additional components and their contents (final concentrations) are as follows: 25 μg / mL of laminin, laminin Adhesin 25 μg / mL, transferrin 0.2 ng / mL, trypsin 10 μg / mL, aprotinin 0.1 mg / mL, growth hormone 20 ng / mL, insulin 1 U / mL, hydrocortisone 0.5 μg / mL, dexamethasone Methasone 0.2nM, bFGF 20ng / mL, EGF 30ng / mL, B27 volume fraction 1%, IGF-I 10ng / mL, IGF-II 30ng / mL, choline 30nM, linoleic acid 10nM, sodium selenite 30nM, phosphoric acid Ethanolamine 50nM, glutathione 2.5μg / mL, vitamin C 50μg / mL, vitamin E 30μg / mL, vitamin B12 10μM, biotin 20μM.
[0016] The specific preparation method is to add the above-mentioned supplementary components to the L-DMEM medium, and make the final concentration to be the above-mentioned concentration, thereby obtaining the serum-free medium for mesenchymal stem cells.
Embodiment 2
[0018] A serum-free medium for mesenchymal stem cells in this embodiment is based on DMEM-F12 medium, and also includes the following additional components, and the additional components and their contents (final concentrations) are as follows: laminin 40 μg / mL, Transferrin 2ng / mL, trypsin 20μg / mL, aprotinin 0.1mg / mL, growth hormone 30ng / mL, insulin 10U / mL, hydrocortisone 1μg / mL, dexamethasone 0.3nM, bFGF 40ng / mL , EGF 20ng / mL, B27 volume fraction 2%, IGF-I 10ng / mL, IGF-II 20ng / mL, choline 30nM, linoleic acid 20nM, sodium selenite 15nM, phosphoethanolamine 30nM, glutathione 5μg / mL, vitamin C 50 μg / mL, vitamin E 30 μg / mL, vitamin B12 20 μM, biotin 30 μM.
[0019] Its specific preparation method is to add the above-mentioned additional components to the DMEM-F12 medium, and make the final concentration to the above-mentioned concentration, thereby obtaining the serum-free medium for mesenchymal stem cells.
Embodiment 3
[0021] A serum-free medium for mesenchymal stem cells in this embodiment uses α-MEM medium as the base medium, and also includes the following additional components, and the additional components and their contents (final concentrations) are as follows: laminin 50 μg / mL, Transferrin 0.2ng / mL, trypsin 30μg / mL, aprotinin 0.1mg / mL, growth hormone 30ng / mL, insulin 6U / mL, hydrocortisone 1μg / mL, dexamethasone 0.3nM, bFGF 30ng / mL mL, EGF 30ng / mL, B27 volume fraction 3%, IGF-I 10ng / mL, IGF-II10ng / mL, choline 20nM, linoleic acid 20nM, sodium selenite 20nM, phosphoethanolamine 20nM, glutathione 2.5 μg / mL, Vitamin C 50μg / mL, Vitamin E 30μg / mL, Vitamin B12 20μM, Biotin 30μM.
[0022] The specific preparation method is to add the above-mentioned supplementary components to the α-MEM medium, and make the final concentration to be the above-mentioned concentration, thereby obtaining the serum-free medium for mesenchymal stem cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com