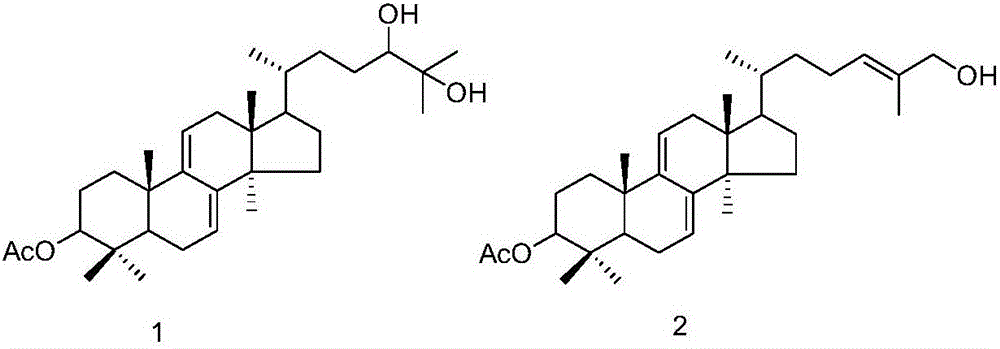
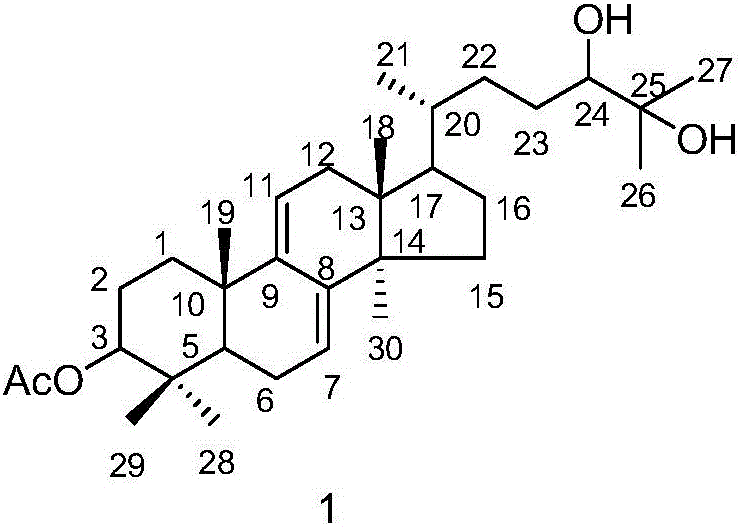
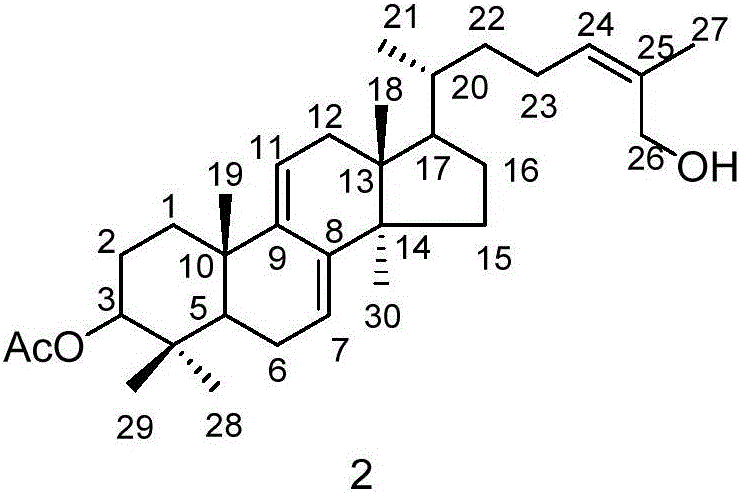
Triterpene compound, and preparation method and applications thereof
A technology of triterpene compound and composition, which is applied in the application field of preparing diabetes treatment drugs, can solve the problems of high adverse reactions and weak activity, and achieve good α-glucosidase inhibitory activity, small molecular weight, and low dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation method of compound 1 and 2
[0027] (1) Ganoderma lucidum dried fruiting body (700g) was pulverized, the powder was soaked and extracted four times with isopropanol (3.5 liters each time, 2h each time), the extracts were combined and concentrated under reduced pressure to obtain the extract (20g).
[0028] (2) Pass the obtained extract through the MCI column (4.5×28cm, the filler is MCI gel CHP20P Mitsubishi Chemical Corporation), and carry out gradient elution with methanol / water solution. The steps of the gradient elution are: sequentially use 40% volume fraction , 50%, 60%, 70%, 80%, 90% methanol / water solution, and 100% methanol were eluted for 2 column volumes respectively.
[0029] (3) Collect the 70% methanol / water eluent in step (2), concentrate under reduced pressure, use 200-300 mesh silica gel as column packing, and carry out gradient elution with petroleum ether / ethyl acetate mixed solution, and the gradient elution The steps o...
Embodiment 2
[0041] Embodiment 2: α-glucosidase inhibitory activity test of compound 1, 2
[0042] The two compounds prepared in Example 1 were formulated into solutions with different concentrations according to their solubility, and the α-glucosidase inhibitory activity was tested.
[0043] First, add α-glucosidase 25uL (0.12U / mL) to the enzyme activity assay system of potassium phosphate buffer (pH 6.8), incubate at 37°C for 15min, add pNPG 25uL (5.0mM), react at 37°C for 30min, add 80uL (1.0M) of sodium carbonate was used to terminate the reaction and measured at 405nm to obtain the absorbance of the blank group. Then take 100uL of acarbose or screening sample and add it to the enzyme activity assay system. First, incubate the enzyme at 37°C for 15min, then add the substrate pNPG, react at 37°C for 30min, add sodium carbonate to terminate the reaction, and measure the p-nitro group at 405nm. The absorbance of phenol. Calculate the inhibition rate of enzyme activity and calculate the ...
Embodiment 3
[0047] Embodiment 3: capsule preparation
[0048] Take 20g of Compound 1, add 79g of lactose monohydrate, 79g of microcrystalline cellulose and 22g of micropowdered silica gel, mix, granulate, and fill into hard capsules to obtain 1000 capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com