A kind of method for preparing difluorobenzene by tubular double nitriding reaction
A technology of nitriding reaction and difluorobenzene, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of low mass transfer and heat transfer efficiency, easy decomposition of diazonium salt, high concentration and temperature, etc. Achieve the effects of reducing three wastes, high product yield, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
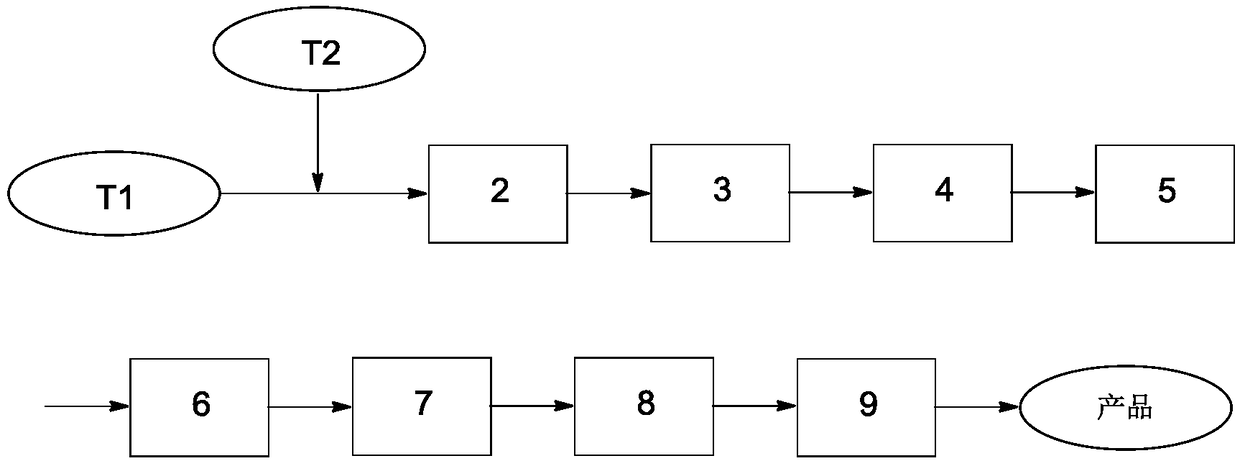
[0025] P-phenylenediamine 10.8kg (100mol), 5% hydrochloric acid aqueous solution 182.3kg (250mol), 20% fluoboric acid aqueous solution 87.8kg (200mol) are mixed and stored in the first storage tank (T1), and sodium nitrite 13.8kg ( 200mol) and 55.2kg of water are stored in the second storage tank (T2), and the two materials are simultaneously fed through the corresponding metering pumps, and the flow rates are adjusted to 18.9L / h and 4.6L / h respectively by the metering pumps. p-Phenylenediamine, HCl in hydrochloric acid aqueous solution, HBF in fluoroboric acid aqueous solution 4 、NaNO 2 The molar flow ratio is 1:2.5:2:2. The material enters the tubular reactor (50m in length and 5mm in diameter) after being mixed by the mixer. The reaction temperature is 0°C and the residence time is 150s. The reaction liquid flows out of the pipe directly Enter the cooling tower, cool to -5°C, filter to obtain a white solid, put it into a cracking kettle after drying, raise the temperature ...
Embodiment 2
[0027] P-phenylenediamine 10.8kg (100mol), 20% hydrochloric acid aqueous solution 273.8kg (1500mol), 50% fluoboric acid aqueous solution 87.8kg (500mol) are mixed and stored in the first storage tank (T1), and sodium nitrite 20.7kg ( 300mol) and 48.3kg of water are stored in the second storage tank (T2), and the two materials are simultaneously fed through the corresponding metering pumps, and the flow rates are adjusted to 40.1L / h and 7.0L / h respectively by the metering pumps. At this time Make p-phenylenediamine, HCl in hydrochloric acid aqueous solution, HBF in fluoroboric acid aqueous solution 4 、NaNO 2 The molar flow ratio is 1:15:5:3. The material enters the tubular reactor (10m in length and 10mm in diameter) after being mixed by the mixer. The reaction temperature is 50°C and the residence time is 60s. The reaction liquid flows out of the pipe directly Enter the cooling tower, cool to -10°C, filter to obtain a white solid, put it into a cracking kettle after drying, r...
Embodiment 3
[0029] P-phenylenediamine 10.8kg (100mol), 37% hydrochloric acid aqueous solution 246.4kg (2500mol), 50% fluoboric acid aqueous solution 70.2kg (400mol) are mixed and stored in the first storage tank (T1), and sodium nitrite 20.7kg ( 300mol) and 20.7kg of water are stored in the second storage tank (T2), and the two materials are simultaneously fed through the corresponding metering pumps, and the flow rates are adjusted to 6387.3L / h and 677.9L / h respectively through the metering pumps. At this time p-Phenylenediamine, HCl in hydrochloric acid aqueous solution, HBF in fluoroboric acid aqueous solution 4 、NaNO 2 The molar flow ratio is 1:25:4:3, the material enters the tubular reactor (tube length 1m, tube diameter 50mm) after being mixed by the mixer, the reaction temperature is 100°C, the residence time is 1s, and the reaction solution flows out of the pipeline directly Enter the cooling tower, cool to -10°C, filter to obtain a white solid, put it into a cracking kettle afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com