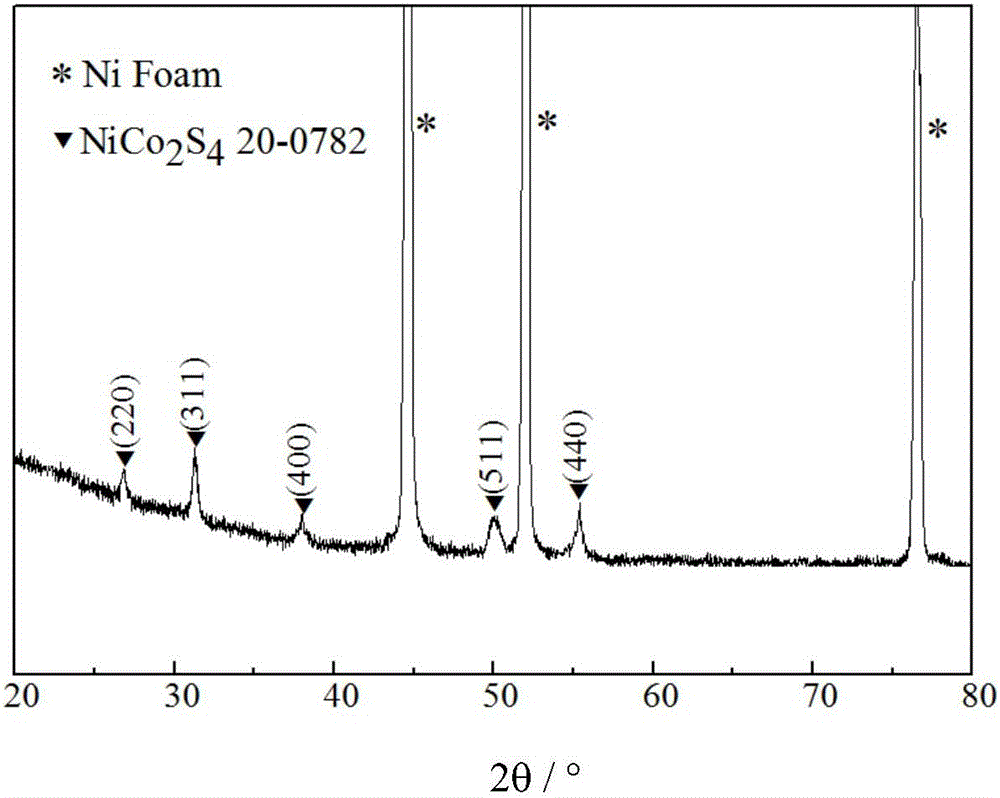
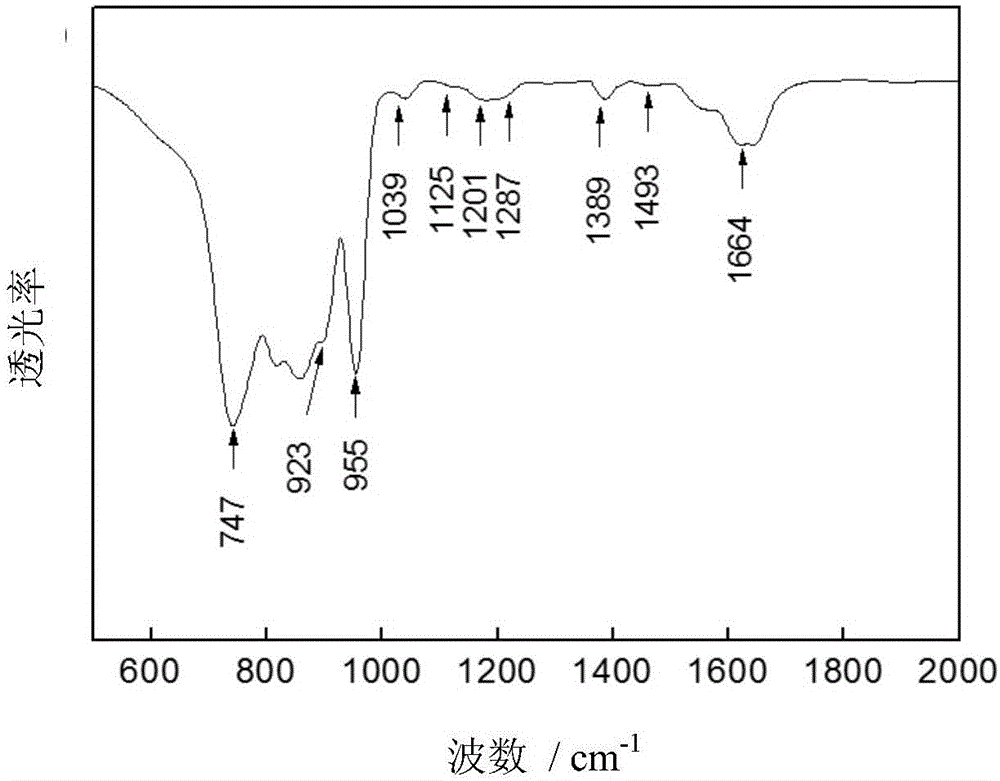
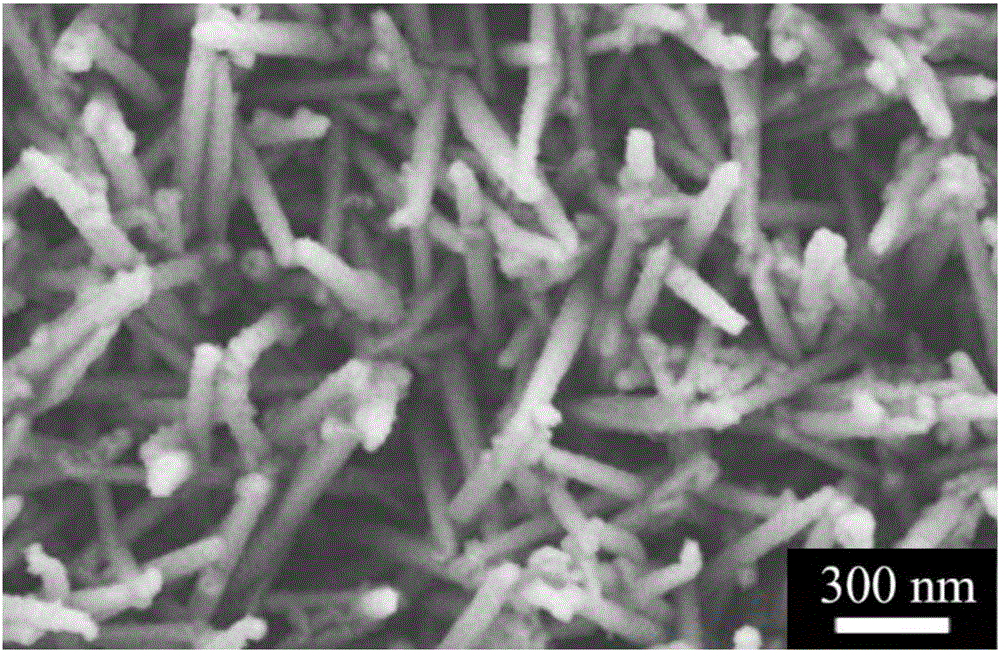
Polypyrrole-coated nickel cobalt sulfide nanotube material, preparation method and applications
A polypyrrole nanometer, cobalt-nickel sulfide technology, applied in the field of materials, can solve the problems of poor structural stability of cobalt-nickel sulfide, lower actual specific capacitance, easy polarization of electrodes, etc., achieve good electrical conduction, high specific capacitance, and improve cycle performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Take 0.47g Ni(NO 3 ) 2 ·6H 2 O, 0.77g CoCl 2 ·6H 2 O, 2.00g of urea and 0.8g of hexamethylenetetramine were dissolved in 80mL of deionized water, and the resulting mixed solution was transferred into a polytetrafluoroethylene reactor with a volume of 100mL, and the cleaned foamed nickel (2×2cm 2 ) into the mixed solution, then seal the reaction vessel, put it into an oven and heat it to 120°C for 6 hours, cool it down to room temperature naturally, take out the nickel foam that has grown the precursor, wash it with deionized water for 5 times, and then dry it at 60°C for 12 hours to obtain Dry nickel foam from the grown precursor.
[0051] Take 0.768g Na 2 S·9H 2 O was dissolved in 80 mL deionized water, and the resulting Na 2 The S solution was moved into a polytetrafluoroethylene reactor with a volume of 100mL, and the dried nickel foam of the grown precursor was immersed in Na 2 S solution, then close the reaction kettle, put it into an oven and heat it to 16...
Embodiment 2
[0067] Take 0.40g Ni(NO 3 ) 2 ·6H 2 O, 0.70g CoCl 2 ·6H 2O, 1.90g of urea and 0.7g of hexamethylenetetramine were dissolved in 79mL of deionized water, and the resulting mixed solution was transferred into a polytetrafluoroethylene reactor with a volume of 100mL, and the cleaned foamed nickel (2×2cm 2 ) into the mixed solution, then seal the reaction vessel, put it in an oven and heat it to 110°C for 5 hours, cool it down to room temperature naturally, take out the nickel foam that has grown the precursor, wash it with deionized water for 5 times, and then dry it at 50°C for 14 hours to obtain Dry nickel foam from the grown precursor.
[0068] Take 0.72g Na 2 S·9H 2 O was dissolved in 80 mL deionized water, and the resulting Na 2 The S solution was moved into a polytetrafluoroethylene reactor with a volume of 100mL, and the dried nickel foam of the grown precursor was immersed in Na 2 S solution, then close the reaction kettle, put it into an oven and heat it to 150°C ...
Embodiment 3
[0077] Take 0.50g Ni(NO 3 ) 2 ·6H 2 O, 0.80g CoCl 2 ·6H 2 O, 2.20g of urea and 0.9g of hexamethylenetetramine were dissolved in 81mL of deionized water, and the resulting mixed solution was transferred into a polytetrafluoroethylene reactor with a capacity of 100mL, and the cleaned foamed nickel (2×2cm 2 ) into the mixed solution, then close the reaction vessel, put it into an oven and heat it to 130°C for 7 hours, cool it down to room temperature naturally, take out the nickel foam that has grown the precursor, wash it with deionized water for 5 times, and then dry it at 70°C for 11 hours to obtain Dry nickel foam from the grown precursor.
[0078] Take 0.832g Na 2 S·9H 2 O was dissolved in 90 mL deionized water, and the resulting Na 2 The S solution was moved into a polytetrafluoroethylene reactor with a volume of 100mL, and the dried nickel foam of the grown precursor was immersed in Na 2 S solution, then close the reaction kettle, put it into an oven and heat it to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com