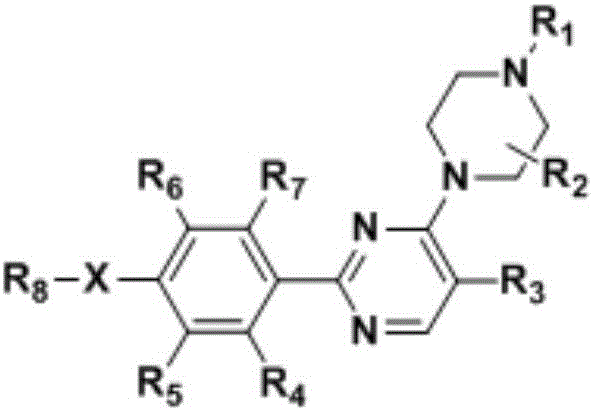
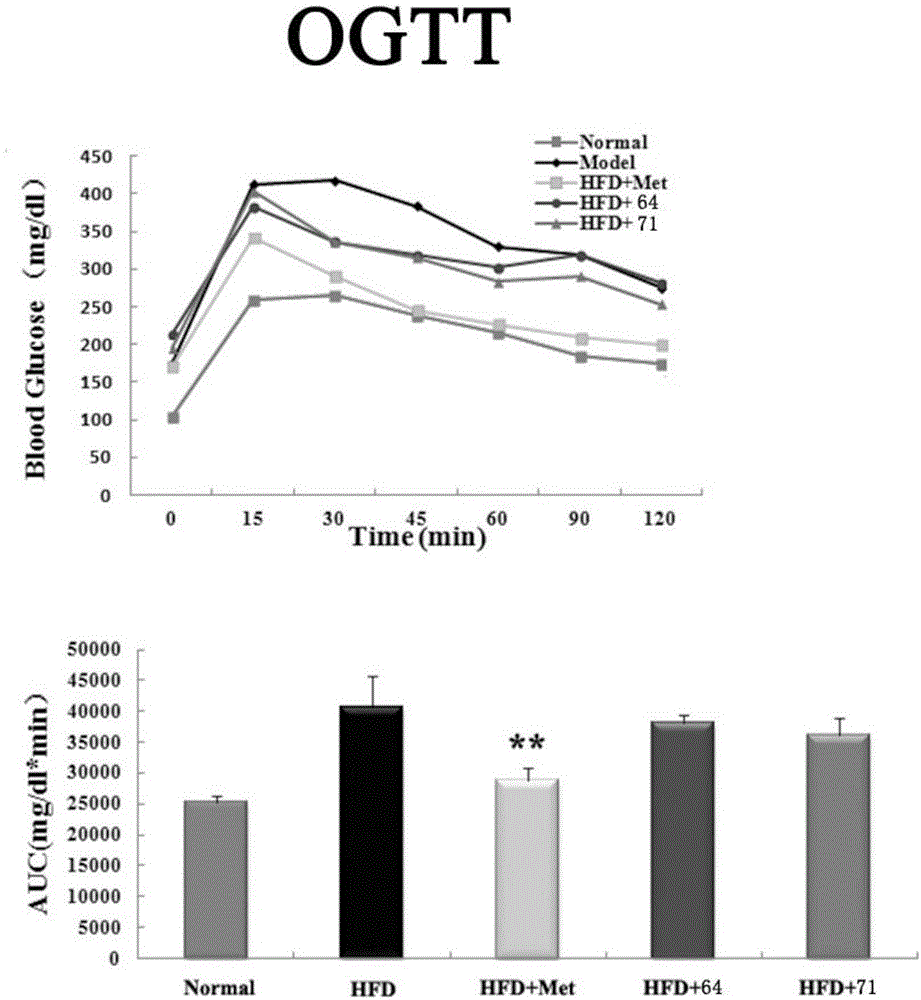
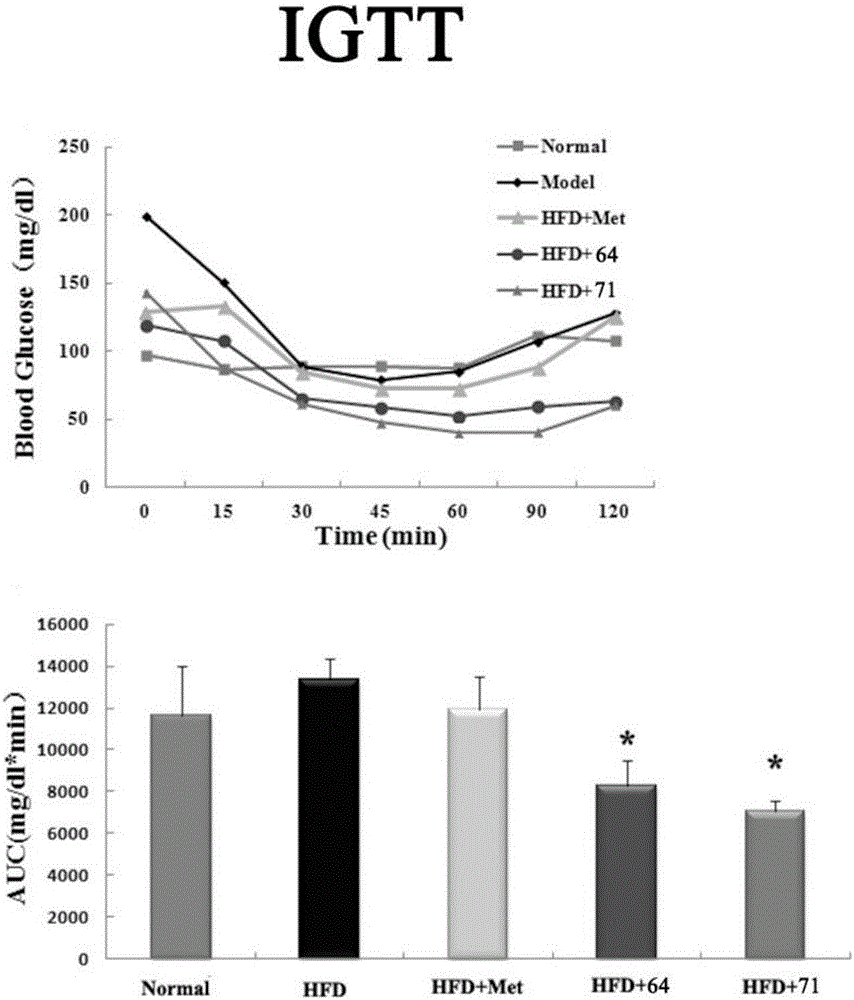
Phenyl pyrimidine derivative and preparation method and use thereof
A technology of methylpyrimidine and methylsulfonylbenzylphenyl, which is applied in the field of phenylpyrimidine derivatives and its preparation, can solve problems that have not yet been discovered, and achieve the effect of increasing glucose consumption, improving glucose tolerance, and obvious effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Boc-4-(2-chloro-5-methylpyrimidine)-piperazine is synthesized
[0037] Put 100mg (0.61mmol) 2,4-dichloropentamethylpyrimidine and 171.39mg (0.92mmol) N-Boc-piperazine in a 100mL round bottom flask, add potassium carbonate 168.36mg (1.2mol) and stir at 50°C Overnight, the reaction solution was poured into ice water, immediately a large amount of white solid precipitated, filtered, washed 2-3 times with ether, and dried to obtain 180.5 mg of white solid, yield 94%.
[0038] The product parameters are as follows: 1 H NMR (400MHz, CDCl3) δ7.94(s,1H),3.82(s,4H),3.50(s,4H),2.25(s,3H),1.43(s,9H).MS(ES),m / z: 313.3 [M+H]+; HPLC purity: 98.0%.
Embodiment 2
[0039] Example 2 Synthesis of N-tert-butoxycarbonyl-4-(2-(4-methoxyphenyl)-5-methylpyrimidine)-4-piperazine
[0040] Put 200mg (0.64mol) of Boc-4-(2-chloro-5-methylpyrimidine)-piperazine and 106.4mg (0.7mol) of p-methoxyphenylboronic acid in a 100mL three-necked bottle, add tetratriphenyl Phosphine palladium 60 mg, seal the bottle mouth with a rubber stopper, vacuumize, and pass in nitrogen gas, ventilate back and forth 3 to 4 times, inject 4 mL of toluene: ethanol = 1:1 solution with a pinhole, 1 mL of 1N sodium carbonate solution, in Stir at 80 degrees Celsius for 6 hours, hang the reacted solution to dry and mix the sample, and purify by column chromatography, eluent conditions: petroleum ether: ethyl acetate = 3:1, dry and hang to give 110 mg of white solid, yield 44.8% .
[0041] The product parameters are as follows: 1 H NMR (400MHz, CDCl3) δ8.30(s,1H),7.93(d,2H,J=6.4Hz),7.39(s,1H),7.04(s,1H),3.82(s,3H),3.50 (s, 8H), 2.25 (s, 3H), 1.43 (s, 9H). MS (ES), m / z: 385.3 [M+...
Embodiment 3
[0042] Example 3 Synthesis of Boc-4-(2-(4-ethoxyphenyl)-5-methylpyrimidine-4-piperazine
[0043] Put 200mg (0.64mol) Boc-4-(2-chloro-5-methylpyrimidine)-piperazine and 116.2mg (0.7mol) p-ethoxyloxyphenylboronic acid in a 100mL three-necked bottle, add four three Phenylphosphine palladium 60 mg, seal the bottle mouth with a rubber stopper, vacuumize, and pass in nitrogen gas, ventilate back and forth 3 to 4 times, inject 4 mL of toluene: ethanol = 1:1 solution with a pinhole, 1 mL of 1N sodium carbonate solution , stirred at 80 degrees Celsius for 6 hours, suspended the reacted solution to dry and mixed samples, purified by column chromatography, eluent conditions: petroleum ether: ethyl acetate = 3:1, dried and suspended to obtain 100.6 mg of a white solid, the product rate 39.4%.
[0044] The product parameters are as follows: 1 H NMR (400MHz, CDCl3) δ8.23(d, 1H, J=8.8Hz), 7.19(s, 1H), 6.89(d, 1H, J=8.8Hz), 7.04(s, 1H), 3.91(m ,2H),3.52(d,8H,J=2.8Hz),2.17(s,3H),1.42(s,9H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com