Oxadiazole phosphinic imide/iridium complex, and preparation method and application thereof
An oxadiazole phosphinic imide iridium complex technology is applied in the field of organic electroluminescence display, which can solve the problems of long luminous life, hinder the application of OLED, poor carrier transport performance, etc., and achieves improved luminous efficiency, The effect of improving the efficiency roll-off phenomenon and improving the carrier transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of complex 1:
[0033] Under anhydrous and oxygen-free conditions, bis-[7,8-benzoquinoline]iridium dichloride (0.50 g, 0.43 mmol) and 2-(N-diphenylphosphinyl)-amino- The potassium salt of 5-phenyl-[1,3,4]-oxadiazole (0.43 g, 1.08 mmol) was dissolved in 15 ml of ethylene glycol monoethyl ether, reacted at 120°C for 12 hours, filtered, and recrystallized. Finally, it was purified by column chromatography to obtain the complex bis[7,8-benzoquinoline]-{2-(N-diphenylphosphoryl)-amino-5-phenyl-[1,3,4] -Oxadiazole} iridium (1).
[0034] Synthesis of complex 2:
[0035] Under anhydrous and oxygen-free conditions, bis-[2-phenylquinoline] iridium dichloride (0.50 g, 0.39 mmol) and 2-(N-diphenylphosphinyl)-amino-5- The potassium salt of phenyl-[1,3,4]-oxadiazole (0.39 g, 0.98 mmol) was dissolved in 15 ml of ethylene glycol monoethyl ether, reacted at 130 ° C for 12 hours, filtered, recrystallized, and finally passed Purified by column chromatography to obtain the com...
Embodiment 2
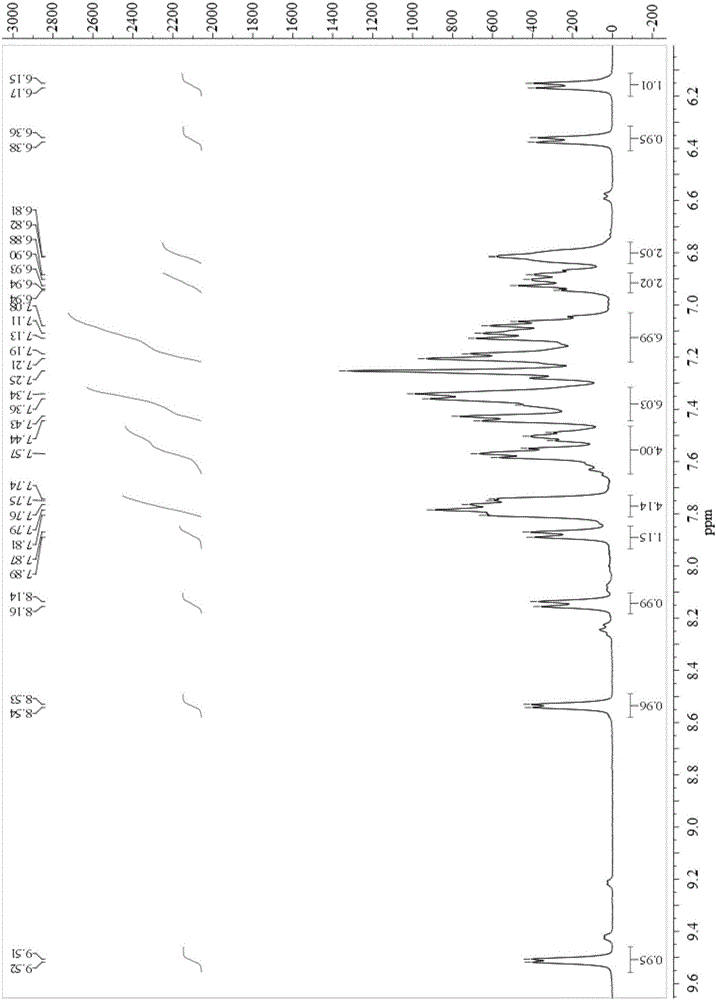
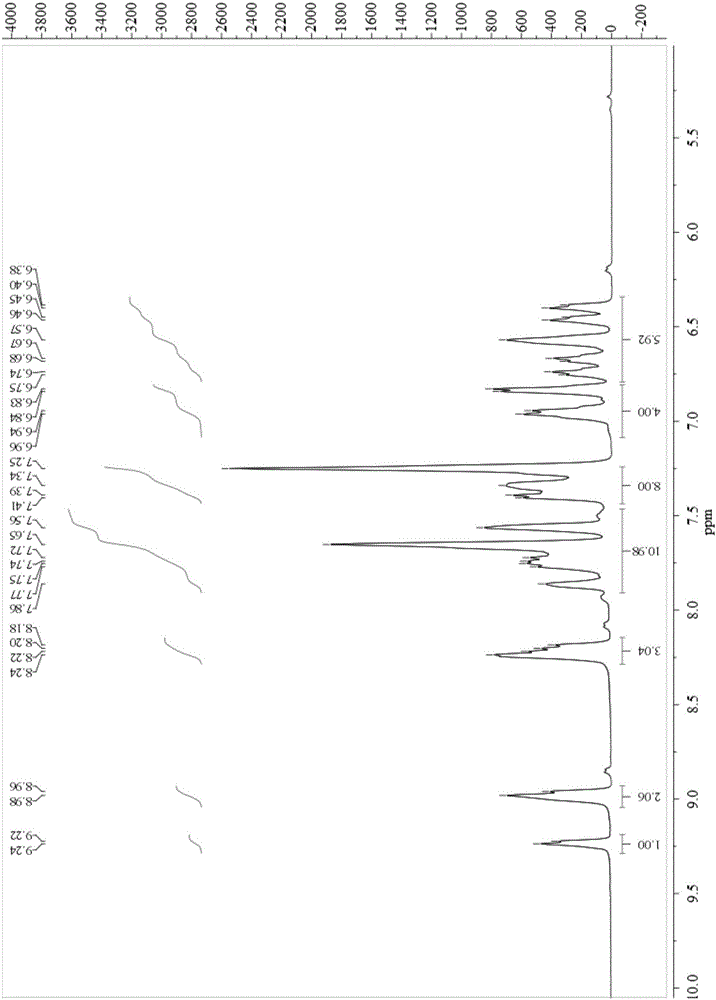
[0055] UV absorption spectra, emission spectra and other characterizations of complexes 1, 2 and 3:
[0056] Complexes 1, 2 and 3 were dissolved in acetonitrile (10 -5 M), oxygen removal, respectively measure its absorption and emission spectra (containing low temperature and thin film emission spectra) on Agilent Cary 60 and Hitachi F-7000 spectrometer:
[0057] The peak positions of the absorption and emission spectra of the complex are:
[0058] Bis[7,8-benzoquinoline]-{2-(N-diphenylphosphinyl)-amino-5-phenyl-[1,3,4]-oxadiazole}iridium(1) :
[0059] lambda abs,max , nm 255, 299, 441 (see attached Figure 4 )
[0060] lambda em,max , nm 539 (acetonitrile solution), 518 (low temperature), 534 (film) (see attached Figure 4 )
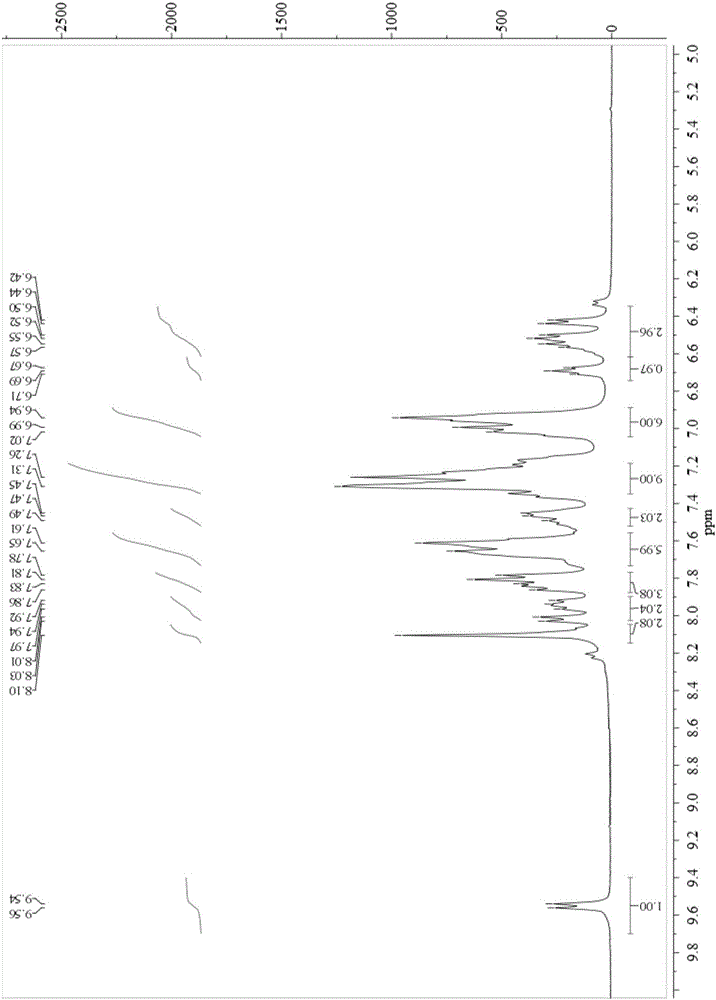
[0061] Bis[2-phenylquinoline]-{2-(N-diphenylphosphinyl)-amino-5-phenyl-[1,3,4]-oxadiazole}iridium (2):
[0062] lambda abs,max , nm 270, 337, 453 (see attached Figure 5 )
[0063] lambda em,max , nm 592 (acetonitrile solution), 575 (low te...
Embodiment 3
[0071] Preparation of organic electroluminescent devices OLEDs with complexes 1, 2 and 3 as luminescent centers:
[0072] Device preparation equipment: MB-MO-SE1 vacuum thermal evaporation coating equipment from Mbraun, Germany; testing equipment: Keithley Source 2400, Photo Research PR650 spectrometer.
[0073] The structure of the device is:
[0074] D1: ITO / HIO2(25nm) / NPB(5nm) / TCTA(10nm) / mCP:Complex 1(20nm) / TPBI(40nm) / Liq(1nm) / Al(100nm)D1
[0075]D2: ITO / HIO2(25nm) / NPB(5nm) / TCTA(10nm) / mCP:Complex 2(20nm) / TPBI(40nm) / Liq(1nm) / Al(100nm)D2
[0076] D3: ITO / HIO2(25nm) / NPB(5nm) / TCTA(10nm) / mCP:Complex 3(20nm) / TPBI(40nm) / Liq(1nm) / Al(100nm)D3
[0077] The current efficiency (cd / A) of the device is obtained from the I-V and L-V characteristics of the device:
[0078] n c = L / I (1)
[0079] The power efficiency of the device can be calculated by the following formula:
[0080] n p =π×S×L / (I×V) (2)
[0081] Wherein, L is the luminous brightness, I is the current density, S is t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com