Cinnamyl aldehyde derivative and application thereof
A derivative, the technology of cinnamaldehyde, applied in the field of cinnamaldehyde derivatives, can solve the problems of easily oxidized water solubility, volatility, irritation, color, temperature, and stability of the formula system, affecting compatibility and availability, etc. , to achieve good antibacterial and bactericidal properties, good compatibility and mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of Example 1 Derivative (1a)
[0060]
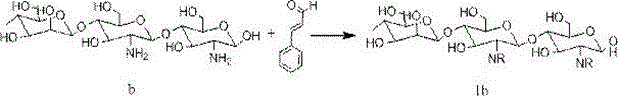
[0061] R: =CH-CH=CH-Ph
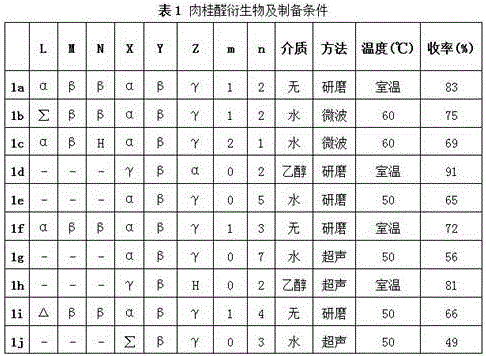
[0062] At room temperature, add 7.8g of cinnamaldehyde (0.06mol) and 5.1g (0.01mol) of compound a into a dry mortar, mix well in the mortar, quickly grind for 20 minutes, and then store in airtight at room temperature for 12 hours, thin After the completion of the reaction monitored by chromatography, add 5.0ml of ethanol (75%), grind for 15 minutes, filter with suction, wash with absolute ethanol three times, and dry the solid in vacuum to obtain a white powdery solid with a yield of 83% (based on raw material a) , Melting point 175°C (decomposition).
Embodiment 2
[0063] Example 2 Preparation of Derivative (1b)
[0064]
[0065] R: =CH-CH=CH-Ph
[0066] At room temperature, add 1.0g of deionized water, 7.8g of cinnamaldehyde (0.06mol) and 5.0g (0.01mol) of compound a into a microwave reactor, irradiate with microwave at 60°C for 10 minutes, cool to room temperature, and monitor the reaction by thin chromatography After completion, add 7.5ml of absolute ethanol, continue to grind for 10 minutes, filter with suction, wash with absolute ethanol three times, and dry the solid in vacuum to obtain a white powdery solid with a yield of 75% (based on raw material b) and a melting point of 161°C ( break down).
Embodiment 3
[0067] Example 3 Preparation of Derivative (1c)
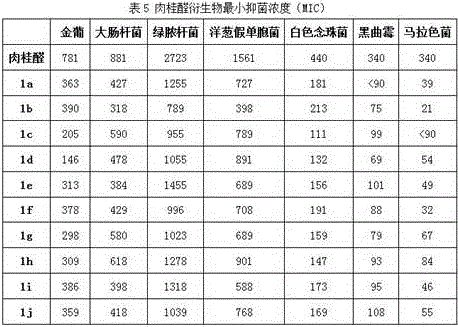
[0068] Preparation was carried out with reference to Example 2, and 1.5 g of water, 3.9 g of cinnamaldehyde (0.03 mol) and 5.1 g (0.01 mol) of compound c were added to the reactor. After post-treatment, a white powdery solid was obtained with a yield of 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com