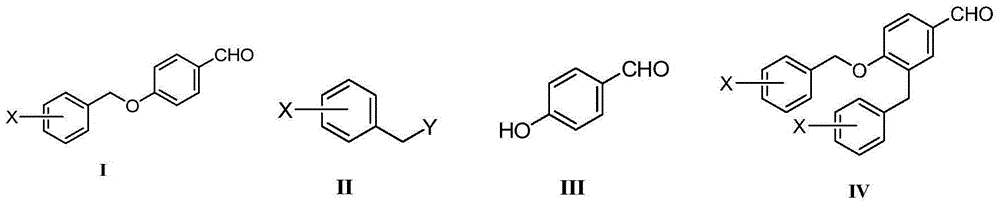
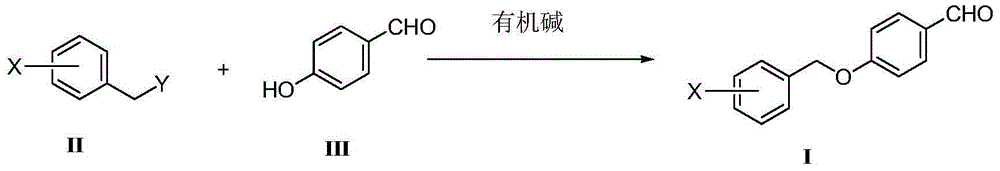
Preparation method of benzyl aryl ether
A benzyl aryl ether and benzyl technology, which is applied in the field of organic compound synthesis, can solve the problems of high content and difficult product purification, and achieves the effects of good economic benefit, high yield and selectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 3-fluorobenzyl chloride II-1 (X=3-F, Y=-Cl) (50g, 0.34mol), p-hydroxybenzaldehyde III-1 (44.3g, 0.36 mol), acetonitrile 500mL, diisopropylethylamine (49.2g, 0.38mol), sodium iodide (5.18g, 0.034mol), after addition, the reaction solution was heated to 50°C and stirred for 24h. Cool to room temperature, concentrate the reaction solution under reduced pressure, add 500 mL of dichloromethane to the residue, wash the resulting mixed solution with 1M hydrochloric acid, saturated sodium carbonate solution and saturated sodium chloride solution successively, dry the organic phase, and concentrate to obtain a crude product. Recrystallization of isopropyl ether gave 71 g of white solid I-1 (X=3-F), yield 89%, Mp: 42.2-43.5°C, purity 99.7% by HPLC, no impurity IV-1 (X=3-F) was detected 3-F).
[0030] 1 H NMR (CDCl 3 ,400MHz) δ9.89(s,1H),7.84(m,2H),7.37(m,1H),7.17(m,2H),7.05(m,3H),5.15(s,2H).
[0031] 19 F NMR (CDCl 3 ,376.5MHz) δ-112.38(s).
Embodiment 2
[0033] Add 3-fluorobenzyl methanesulfonate II-2 (X=3-F, Y=CH 3 SO 3 -) (69.4g, 0.34mol), p-hydroxybenzaldehyde III-1 (44.3g, 0.36mol), tetrahydrofuran 500mL, triethylamine (38.5g, 0.38mol), sodium iodide (5.18g, 0.034mol), After the addition, the reaction solution was stirred at 20-25°C for 24h. Concentrate the reaction solution under reduced pressure, add 500 mL of dichloromethane to the residue, wash the resulting mixed solution with 1M hydrochloric acid, saturated sodium carbonate solution and saturated sodium chloride solution successively, dry the organic phase, and concentrate to obtain a crude product, which is reconstituted with isopropyl ether. Crystallization gave 70 g of white solid I-1 (X=3-F), yield 87.7%, Mp: 42.5-43.7°C, purity 99.72% by HPLC, no impurity IV-1 (X=3-F) was detected .
[0034] 1 H NMR spectrum and 19 The F NMR spectral data are consistent with Example 1.
Embodiment 3
[0036] Add 3-fluorobenzyl chloride II-1 (X=3-F, Y=-Cl) (25g, 0.17mol), p-hydroxybenzaldehyde III-1 (22.2g, 0.18 mol), methanol 250mL, DBU (28.8g, 0.19mol), sodium iodide (2.59g, 0.017mol), after addition, the reaction solution was stirred at 20-25°C for 24h. Concentrate the reaction solution under reduced pressure, add 250 mL of dichloromethane to the residue, wash the resulting mixed solution with 1M hydrochloric acid, saturated sodium carbonate solution and saturated sodium chloride solution successively, dry the organic phase, and concentrate to obtain a crude product, which is reconstituted with isopropyl ether. Crystallization gave 35.9 g of white solid I-1 (X=3-F), yield 90%, Mp: 42.6-43.8°C, purity 99.79% by HPLC, no impurity IV-1 (X=3-F) was detected ).
[0037] 1 H NMR spectrum and 19 The F NMR spectrum data are consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com