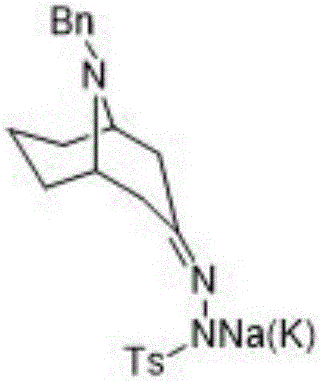
Preparation method of 2-azanoradamantane-N-Oxyl
A non-adamantane, free radical technology, applied in the field of preparation of Nor-AZADO, can solve the problems of not strictly optimized reaction conditions parameters, limited application and promotion, too many acidic wastewater, etc., and achieves easy industrialized large-scale production and great economic value. and social values, mild and safe effects of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
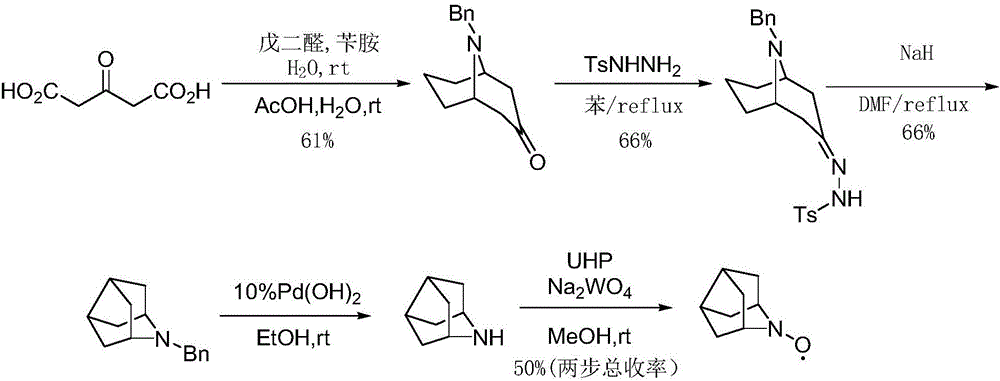
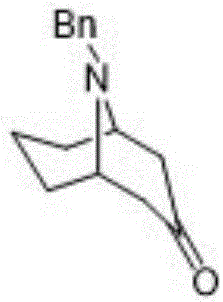
[0048] In said step 1, the compound 9-benzyl-9-azabicyclo-[3,3,1]nonan-3-one was prepared according to the following scheme:
[0049] Benzylamine (5.0g, 47mmol) and Na 2 HPO 4 12H 2 O (5.0g) was dissolved in 50ml of water, acetone dicarboxylic acid (3.5g, 24mmol, 1.2eq) was added, and a concentration of 25% glutaraldehyde (8g, 20mmol, 1.0eq) was added dropwise in an ice-water bath, and maintained After 60 minutes, rise to room temperature and react for 24 hours, then dropwise add concentrated hydrochloric acid to adjust the pH value to 3, stir and react in an oil bath at 50°C for 2 hours, cool to room temperature, and adjust the pH value to 10 with 20% NaOH solution. Then extract twice with dichloromethane, combine the organic phases, wash the organic phase once with saturated brine, and concentrate under reduced pressure to obtain 9-benzyl-9-azabicyclo-[3,3,1]nonan-3-one soil The crude yellow solid was crystallized and purified with ethyl acetate and petroleum ether at a v...
example 2
[0051] In said step 1, the compound 9-benzyl-9-azabicyclo-[3,3,1]nonan-3-one was prepared according to the following scheme:
[0052] Benzylamine (50g, 1.2eq) and Na 2 HPO 4 12H 2 O (40.83g, 0.4eq) was dissolved in 400mL of water, acetone dicarboxylic acid (35g, 240mmol, 1.2eq) was added to adjust the pH value to 4.8-5.2, and a concentration of 25% glutaraldehyde ( 80g, 200mmol, 1eq), and maintained for 30 minutes, raised to room temperature and reacted for 24 hours, added dropwise concentrated hydrochloric acid to adjust the pH value to 3, then stirred and reacted in 70°C oil bath for 1 hour, after the reaction was cooled to room temperature, used 20% NaOH solution adjusted the pH value to 10, filtered, the filtrate was extracted twice with dichloromethane, the organic phases were combined, the organic phase was washed once with saturated brine, and concentrated under reduced pressure to obtain a reddish-brown oil, with a volume ratio of 1: 5 ethyl acetate and petroleum et...
example 3
[0054] In the step 1, the compound 9-benzyl-9-azabicyclo-[3,3,1]nonan-3-one was prepared according to the following scheme.
[0055] Add 400g of disodium hydrogen phosphate, 300g of citric acid, and 3.2Kg of deionized water into a 10L reaction bottle, stir at room temperature for 20 minutes, use ice salt to adjust the temperature to about 5°C, and then add 1000g of glutaraldehyde with a concentration of 25% at one time , control the reaction temperature below 10°C, add 537g of benzylamine hydrochloride, adjust the pH value between 4.5-5.5, add 500g of acetone dicarboxylic acid in batches, control the reaction temperature at about 10°C, and a small amount of bubbles will be generated. After the addition is complete, use 20% NaOH solution to adjust the pH value to about 4.8-5.2, stir overnight at room temperature, add concentrated hydrochloric acid dropwise to adjust the pH value to 2-3, then stir and react in an oil bath at 55°C for 1 hour, and wait for the reaction to cool Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com