Antigen epitope peptide and application thereof
An antigen epitope and antigen technology, applied in the field of genetic engineering and biomedicine, can solve the problems of low tumor killing efficiency, less secretion of cytokines, poor antigen presentation effect, etc., and achieve excellent killing effect, convenient synthesis, chemical properties and The effect of stabilizing thermodynamic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] An embodiment provided by the present invention is prepared by artificial synthesis. The preparation method step of described artificial synthesis is:
[0041] Firstly, a synthetic epitope peptide (shown in SEQ ID NO: 1). The synthesis method is as follows: the HBV core epitope peptide and the HBV surface antigen epitope peptide are connected in series to obtain the epitope peptide (SEQ ID NO: 1). The HBV core epitope peptide is preferably HBcAg and / or HBsAg, and the present invention takes HBcAg18-27 (shown in SEQ ID NO: 2) as an example. The HBV surface antigen epitope peptide is preferably HBV Pre-S2, and the present invention takes HBV Pre-S2 44-53 (shown in SEQ ID NO: 3) as an example.
[0042] The present invention entrusts Nanjing GenScript Biotechnology Co., Ltd. to synthesize the HBV core epitope peptide HBcAg (SEQ ID NO: 2), the surface antigen epitope peptide HBV Pre-S2 (SEQ ID NO: 3) and The antigenic epitope peptide (SEQ ID NO: 1), and its sequence and m...
Embodiment 1
[0065] Example 1 Epitope Peptide Loaded DCs
[0066] 1) Preparation of epitope peptide
[0067] The present invention entrusts Nanjing GenScript Biotechnology Co., Ltd. to synthesize HBV core epitope peptide HBcAg (SEQ ID NO: 2), surface antigen epitope peptide HBV Pre-S2 (SEQ ID NO: 3) and antigen epitope peptide (SEQ ID NO: 3) NO: 1), and its sequence and molecular weight were identified by high-performance liquid chromatography and mass spectrometry, and the concentration was above 95%.
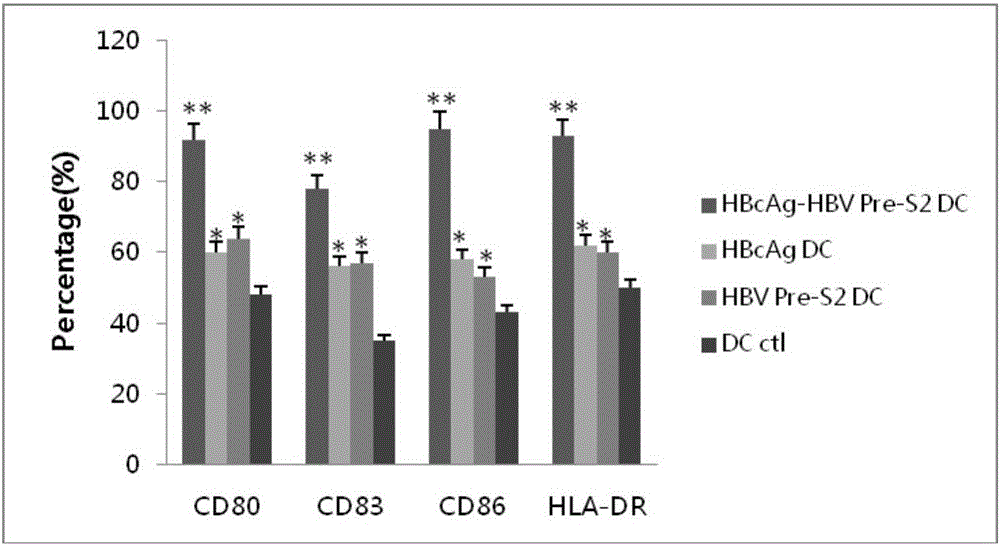
[0068] 2) Epitope peptide-loaded DCs
[0069] Depending on the solubility of the epitope peptide, it was dissolved in water or DMSO, and the concentration of the stock solution was 10 mg / ml. Sterilize by filtration with a 0.22 μm syringe filter, store at -20°C after aliquoting. When loading DCs with epitope peptides, the final concentration of epitope peptides was 40 μg / ml.
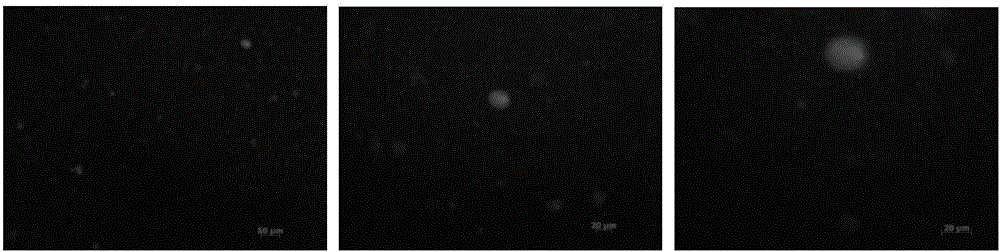
[0070] 3) Loading DCs with epitope peptides linked with FITC fluorescent groups
[0071] Experimental results ...
Embodiment 2D
[0072] The cultivation of embodiment 2DCs and CTL
[0073] (1) Take peripheral blood, add heparin for anticoagulation, then add ficoll (1.077g / ml) for density gradient centrifugation (20°C, 1500r / min, 15min), take interfacial cells, wash twice with PBS, 400r / min , 10min, wash 2 times. Cells were collected and placed in a 6-well plate filled with AIM-V medium and incubated in a carbon dioxide incubator for 1.5-2.0 h. Gently take out the 6-well plate, aspirate the supernatant and suspended cells into another culture dish for later use.
[0074] (2) Add culture medium and rhGM-CSF 800IU / ml, IL-4500IU / ml to the culture wells filled with adherent cells, culture in a carbon dioxide incubator, add cytokines once after 48h, and add TNF-α250IU on the fifth day of culture / ml, continue to culture, DC cells mature on the 7th day.
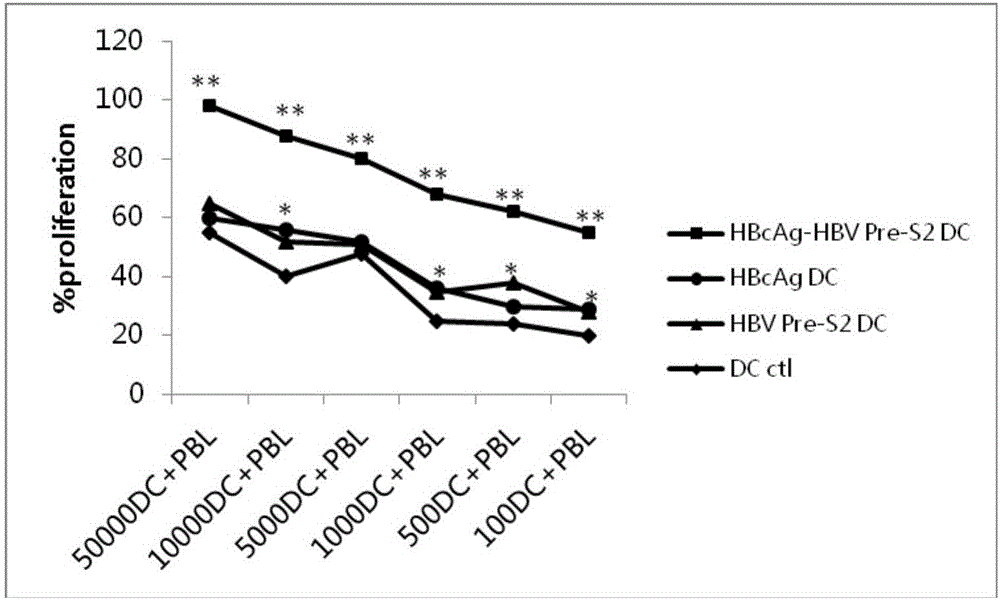
[0075] (3) Culture of T lymphocytes and induction of DC-CTL in vitro
[0076] The suspension cells in step (2) were cultured as T cells, and the cell conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Cell concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com