A troponin I monoclonal antibody magnetic particle and its preparation method and detection kit
A monoclonal antibody and detection kit technology, applied in the field of in vitro diagnostic medical testing, can solve the problems of poor clinical application prospects, poor repeatability, unfavorable promotion of troponin I detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
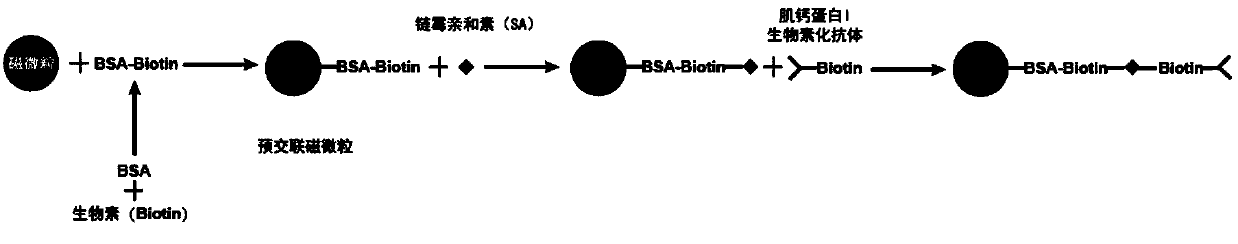
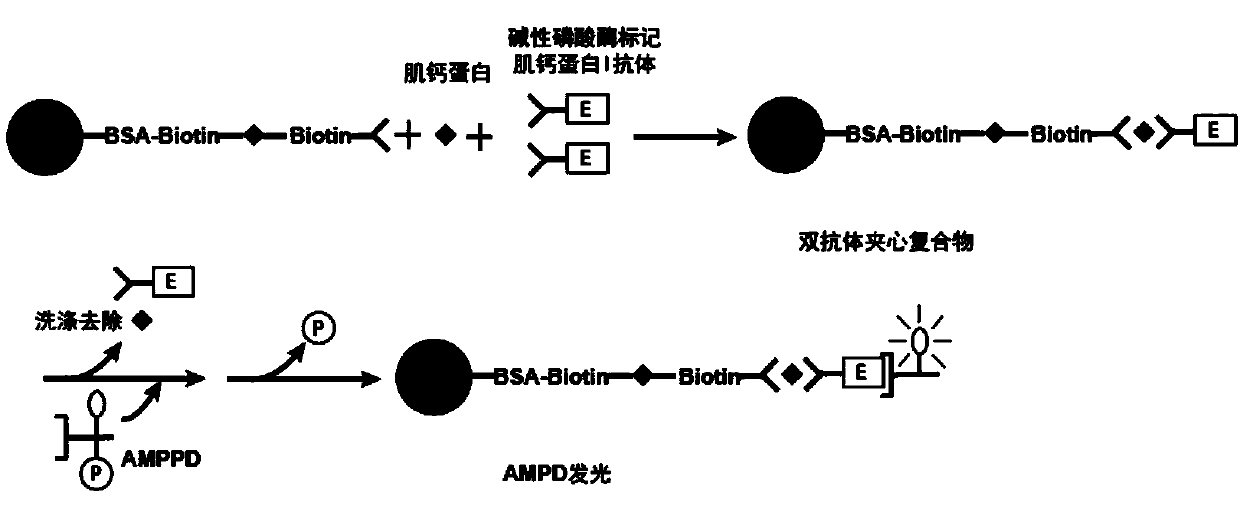
[0063] Correspondingly, the present invention also provides a method for preparing the troponin I monoclonal antibody magnetic particles described above, the method comprising the following steps:
[0064] 1) Using biotin derivatives to pre-crosslink magnetic particles;
[0065] 2) coating the biotin derivative pre-crosslinked magnetic particles obtained in step 1) with streptavidin; and
[0066] 3) Blocking the magnetic particles obtained in step 2), and then reacting with troponin I monoclonal antibody-biotin to prepare troponin I monoclonal antibody magnetic particles.
[0067] In specific embodiments, the method may include:
[0068] a) using biotin derivatives to pre-crosslink the magnetic particles, and then coating them with streptavidin;
[0069] b) reacting the magnetic particles treated in step a) with troponin I monoclonal antibody-biotin after being blocked;
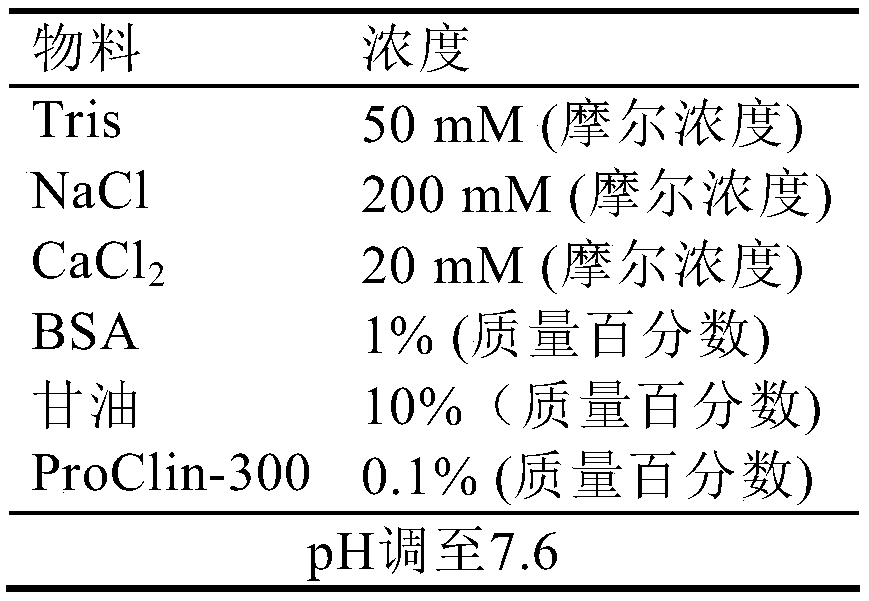
[0070] c) Preserving the magnetic particles treated in step b) in a magnetic particle preservation solu...
Embodiment 1
[0142] Example 1. Preparation of Troponin I Monoclonal Antibody Magnetic Particles
[0143] 1) Dissolve and dilute BSA with 10mM PBS to 2mg / mL, add 2mg / mL Biotin aqueous solution to the 2mg / mL BSA solution according to the molar concentration ratio of 1:20, and mix well;
[0144] 2) React the mixture at room temperature for 30 minutes, place it at 2-8°C, and dialyze with 10mM PBS for 16-24 hours;
[0145] 3) Take out the dialysate and dilute BSA-Biotin to 1mg / mL with 10mM PBS;
[0146] 4) Take 10 mg of 1.0 μm magnetic particles whose active functional groups are carboxyl groups, and wash them twice with 50 mM MES buffer at pH 6.0;
[0147] 5) Remove the supernatant after magnetic suction, add 0.5mL of 50mM MES buffer solution with pH 6.0 and mix well, then add 0.5mL of 25mg / mL carbodiimide (EDC) solution, mix well, and react at room temperature for 30 minutes;
[0148] 6) Wash twice with a solution containing 50 mM pH 7.8 Tris-HCl, 150 mM NaCl, and 1% BSA;
[0149] 7) Add 4...
Embodiment 2
[0161] Example 2. Sensitivity Detection of Troponin I Monoclonal Antibody Magnetic Particles
[0162] Using the troponin I monoclonal antibody magnetic particles obtained in Example 1, the sensitivity detection was carried out as described in the materials and methods, and the results are shown in the following table:
[0163]
[0164]
[0165] Note:
[0166] (1) Magnetic particles obtained by directly cross-linking troponin I monoclonal antibody;
[0167] (2) Traditional streptavidin-biotin troponin I monoclonal antibody magnetic particles;
[0168] (3) The multistage amplified streptavidin-biotin troponin I monoclonal antibody magnetic particle of the present invention.
[0169] As can be seen from the above table, using the multistage amplified streptavidin-biotin troponin I monoclonal antibody magnetic particles of the present invention, its sensitivity (S / N value) is higher than that of direct cross-linking and traditional streptavidin - The biotin method improve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com