Preparation method of magnetic chitosan compound adsorbent
A magnetic adsorbent and composite adsorption technology, applied in chemical instruments and methods, adsorption water/sewage treatment, alkali metal compounds, etc., can solve the problems of easy aggregation of nanoparticles, increase in particle size, and easy corrosion by the external environment, etc. Achieve the effects of avoiding magnetic nucleus agglomeration, improving stability, and excellent selective adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
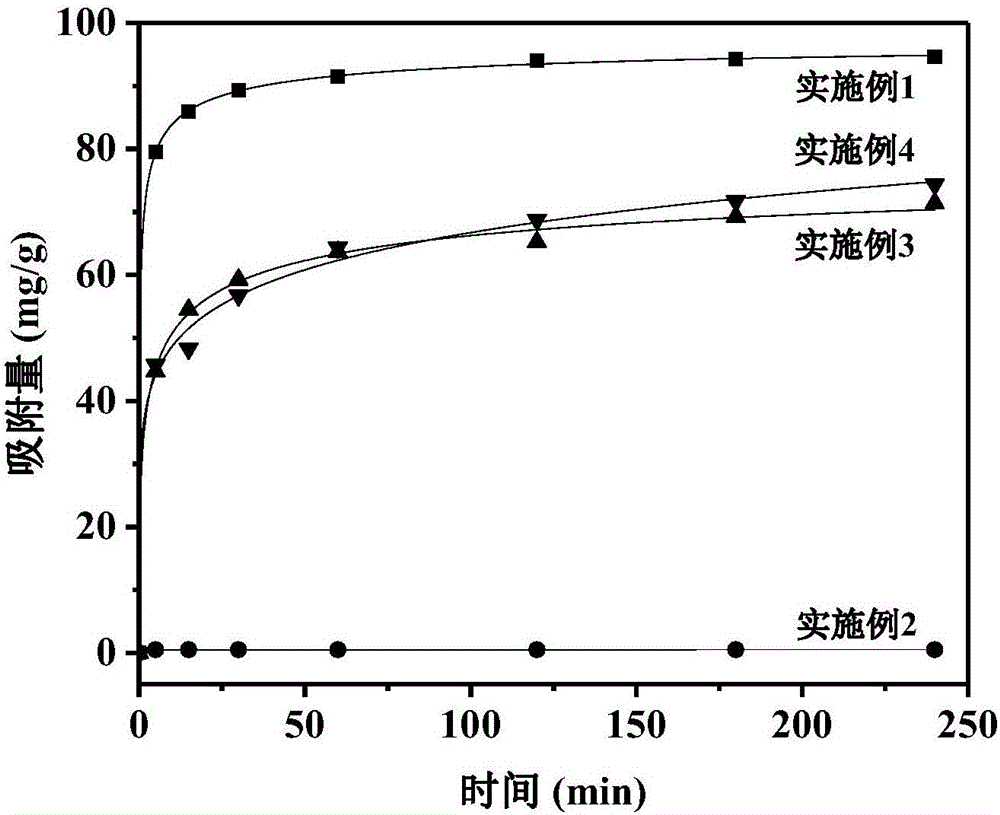
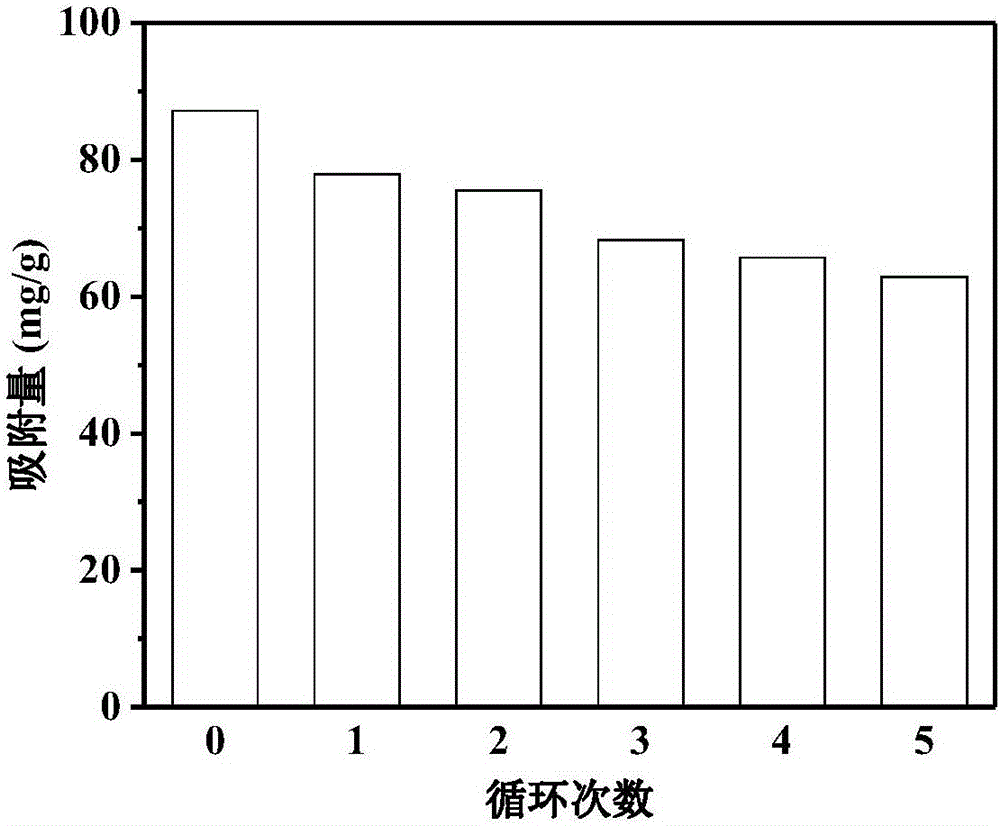
Examples
Embodiment 1
[0033] (1) Spherical magnetic core Fe 3 o 4 Preparation of:
[0034] First weigh 2.5g FeCl successively at room temperature 3 ·6H 2 O, 4g NaAc and 1g polyethylene glycol 2000, and then dissolved in 60ml of ethylene glycol, stirred magnetically until a brownish yellow solution was formed, transferred the obtained solution to a 100ml hydrothermal kettle, and reacted at 200°C for 24h; After cooling to room temperature, the black suspension was washed three times with deionized water and absolute ethanol, and the black solid was separated by a permanent magnet, and then dried in vacuum at 60°C for 12 hours to obtain a spherical magnetic core Fe 3 o 4 .
[0035] (2) Composite magnetic carrier Fe 3 o 4 @Al 2 o 3 Preparation of:
[0036] Sonicate in a water bath for 15 minutes, and place 0.2 g of spherical magnetic core Fe at room temperature 3 o 4 Evenly disperse in 120ml, 0.05mol / L ethanol solution of aluminum isopropoxide, add 60ml ethanol solution with absolute ethano...
Embodiment 2
[0042] (1) Preparation of spherical magnetic core
[0043] Magnetic core Fe 3 o 4 The preparation steps are the same as in Example 1.
[0044] (2) Preparation of Composite Magnetic Carrier
[0045] Sonicate in a water bath for 15 minutes, and place 0.2 g of spherical magnetic core Fe at room temperature 3 o 4 Evenly disperse in 120ml, 0.033mol / L ethanol solution of aluminum isopropoxide, add 60ml of ethanol solution with a volume ratio of absolute ethanol and deionized water of 5:1, and then magnetically stir for 6h to hydrolyze aluminum isopropoxide into Pseudoboehmite and coated in the magnetic core Fe 3 o 4 The surface was washed three times with deionized water and absolute ethanol, and dried under vacuum at 60°C for 12 hours. 2 After calcination at 500°C for 4 hours under the protection of atmosphere, the magnetic carrier Fe 3 o 4 @Al 2 o 3 .
[0046] (3) Preparation of chitosan-coated magnetic adsorbent
[0047] First, 2g of thiourea and 6ml of 25wt% glutarald...
Embodiment 3
[0050] (1) Preparation of spherical magnetic core
[0051] Magnetic core Fe 3 o 4 The preparation steps are the same as in Example 1.
[0052] (2) Preparation of Composite Magnetic Carrier
[0053] Sonicate in a water bath for 15 minutes, and place 0.2 g of spherical magnetic core Fe at room temperature 3 o 4 Uniformly disperse in 120ml, 0.066mol / L ethanol solution of aluminum isopropoxide, add absolute ethanol and deionized water volume ratio of 5:1 60ml ethanol solution, and then magnetically stir for 6h to hydrolyze aluminum isopropoxide into Pseudoboehmite and coated in the magnetic core Fe 3 o 4 The surface was washed three times with deionized water and absolute ethanol, and dried under vacuum at 60°C for 12 hours. 2 After calcination at 500°C for 4 hours under the protection of atmosphere, the magnetic carrier Fe 3 o 4 @Al 2 o 3 .
[0054] (3) Preparation of chitosan-coated magnetic adsorbent
[0055] First, 2g of thiourea and 6ml, 25wt% of glutaraldehyde s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com