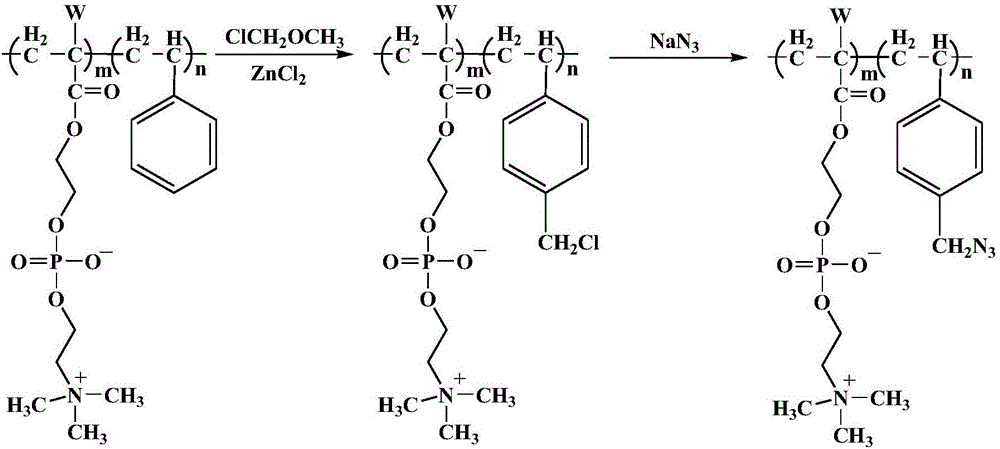
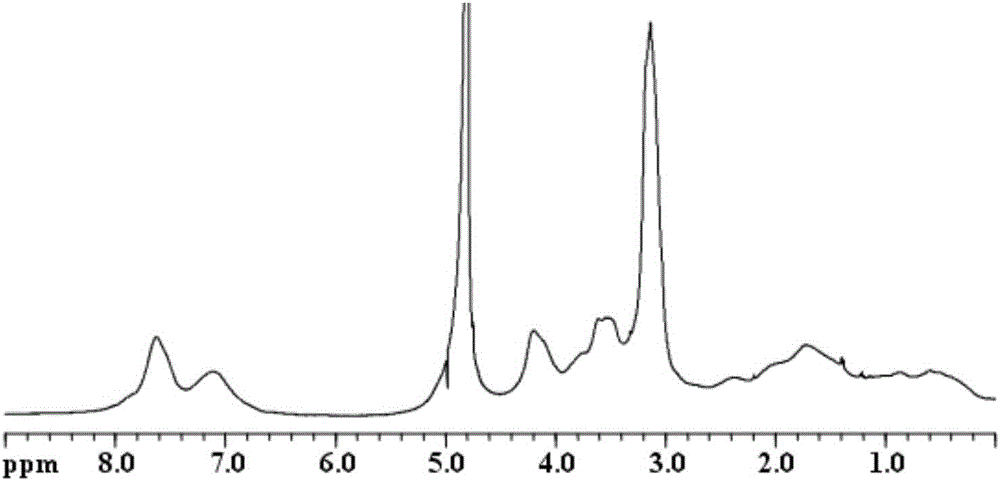
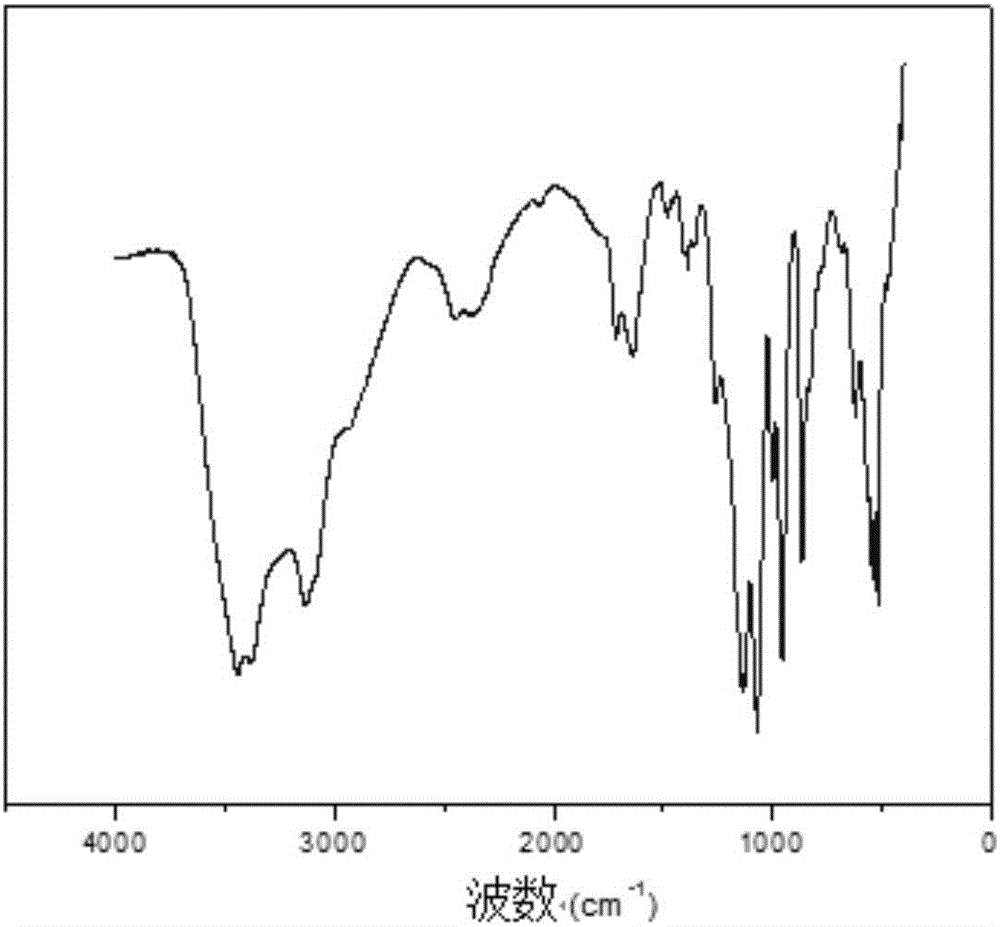
Preparation method of photocurable phosphorylcholine polymer
A phosphorylcholine and polymer technology, which is applied in the field of preparation of photocurable phosphorylcholine polymers, can solve problems such as shedding, and achieve the effects of mild conditions, broad application prospects, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of the photocurable phosphorylcholine polymer of the present embodiment comprises the following steps:
[0031] Step 1. Dissolve 10mmol 2-methacryloyloxyethylphosphorylcholine and 10mmol styrene in methanol and mix them uniformly to obtain a mixed solution of monomers. 2 Protection, preheat the mixed solution of monomers at 70°C for 30 minutes, then add 0.1 mmol of azobisisobutyronitrile, react for 20 hours at 70°C with stirring, concentrate the reaction solution after the reaction, and use a molecular weight cut-off of 6000-8000D The dialysis bag was dialyzed, and the dialyzed sample was freeze-dried at -50°C to obtain a phosphorylcholine polymer containing a benzene ring;
[0032] Step 2. Dissolve 0.2 g of the phenyl ring-containing phosphorylcholine polymer described in step 1 with 30 mL of a mixed solvent, then add 0.2 g of zinc chloride and 5 mL of chloromethyl ether to the dissolved solution, and at 40° C. After reacting for 15 hours, filte...
Embodiment 2
[0037] The preparation method of the photocurable phosphorylcholine polymer of the present embodiment comprises the following steps:
[0038] Step 1. Dissolve 6mmol of acryloyloxyethylphosphorylcholine and 14mmol of styrene in methanol and mix uniformly to obtain a mixed solution of monomers. 2 Protection, preheat the mixed solution of the monomers under the condition of stirring at 75°C for 30min, then add 0.1mmol of azobisisobutyronitrile to react, under the condition of stirring at 75°C for 20h, concentrate the reaction solution after the reaction, and use a molecular weight cut-off of 6000~8000D The dialysis bag was dialyzed, and the dialyzed sample was freeze-dried at -50°C to obtain a phosphorylcholine polymer containing a benzene ring;
[0039] Step 2. Dissolve 0.2 g of the phenyl ring-containing phosphorylcholine polymer described in step 1 with 30 mL of a mixed solvent, then add 0.2 g of zinc chloride and 5 mL of chloromethyl ether to the dissolved solution, and at 40° ...
Embodiment 3
[0043] The preparation method of the photocurable phosphorylcholine polymer of the present embodiment comprises the following steps:
[0044] Step 1. Dissolve 8mmol 2-methacryloyloxyethylphosphorylcholine and 12mmol styrene in methanol and mix them uniformly to obtain a mixed solution of monomers. 2 Protection, preheat the mixed solution of monomers at 80°C for 30 minutes, then add 0.1 mmol of azobisisobutyronitrile, react at 80°C for 12 hours, concentrate the reaction solution after the reaction, and use a molecular weight cut-off of 6000-8000D The dialysis bag was dialyzed, and the dialyzed sample was freeze-dried at -50°C to obtain a phosphorylcholine polymer containing a benzene ring;
[0045] Step 2. Dissolve 0.2 g of the phenyl ring-containing phosphorylcholine polymer described in step 1 with 30 mL of a mixed solvent, then add 0.2 g of zinc chloride and 5 mL of chloromethyl ether to the dissolved solution, and dissolve at 30° C. After reacting for 10 hours, filter, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com