Method for preparing diol mono-tert-butyl ether
A mono-tert-butyl ether and diol technology, which is applied in the field of diol mono-tert-butyl ether preparation, can solve the problems of reduced reactor efficiency, complicated operation, and high equipment investment, and achieve equipment investment and energy consumption reduction, The effect of the simplification of the separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of ethylene glycol mono-tert-butyl ether.
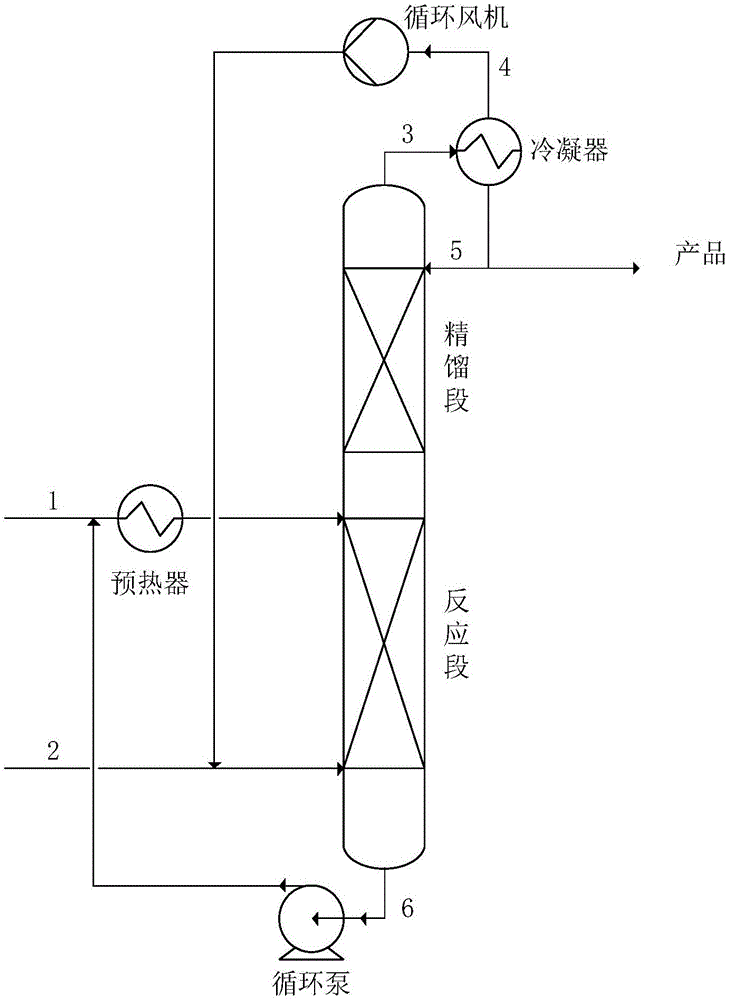
[0028] The reactor is a reactive distillation tower, including a rectification section and a reaction section, the material is 316L, the inner diameter is 65mm, the height of the catalyst bed in the reaction section is 1m, and the height of the rectification section is 1m. The reaction section is filled with acidic resin (Amberlyst-70, Dow Chemical) soaked and replaced with ethylene glycol in advance. The lower part of the bed is supported by a sieve plate and the glass fiber cloth laid on it, and the upper part of the bed is supported by glass fiber cloth and its The upper sieve plate is fixed, and the stainless steel corrugated wire mesh structured packing is used in the middle of the bed, and the resin is dispersed and filled in the gaps of the corrugated wire mesh structured packing. The porosity of the structured packing is 95%, and the specific surface area is 700m 2 / m 3 , the interlayer spacing is 5mm, th...
Embodiment 2
[0032] Preparation of propylene glycol mono-tert-butyl ether.
[0033] The reactor was the same as in Example 1.
[0034] The reactor is operated under normal pressure, 1,2-propanediol is preheated to 170°C through the preheater from the fresh glycol pipeline 1 and fed to the top of the reaction section, and isobutene is fed into the reaction in gaseous form from the fresh isobutene pipeline 2 At the bottom of the section, liquid 1,2-propanediol and gaseous isobutene flow countercurrently in the catalyst bed, and the bed temperature is maintained at 170°C. The unreacted 1,2-propanediol (boiling point 188°C) enters the tower tank, and circulates back to the front end of the preheater through the circulation pump and the glycol circulation line 6 . The 1,2-propanediol mono-tert-butyl ether (boiling point 143° C.) and unreacted isobutylene generated by the reaction escape from the reaction section, and after being purified by the rectification section, the top extraction pipelin...
Embodiment 3
[0037] Preparation of ethylene glycol mono-tert-butyl ether.
[0038] The reactor is a reactive distillation tower, including a rectification section and a reaction section, the material is 316L, the inner diameter is 65mm, the height of the catalyst bed in the reaction section is 1m, and the height of the rectification section is 1.5m. The reaction section is filled with acidic resin (Amberlyst-70, Dow) soaked and replaced with ethylene glycol in advance. The lower part of the bed is supported by a sieve plate and the glass fiber cloth laid on it, and the upper part of the bed is supported by glass fiber cloth and its upper part. The sieve plate is fixed, and the stainless steel corrugated wire mesh structured packing is used in the middle of the bed, and the resin is dispersed and filled in the gaps of the corrugated wire mesh structured packing. The porosity of the structured packing is 80%, and the specific surface area is 400m 2 / m 3 , the interlayer spacing is 8mm, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com