LCZ696 crystallized powder and a preparing method thereof
A technology of crystalline powder and solvent, applied in the field of medicine, can solve the problems of slow suction filtration, sticky product, failure to achieve LCZ696 formulation formulation and process optimization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Preparation of AHU377 free acid:
[0078] Add 100g of AHU377 calcium salt and 1000ml of isopropyl acetate into a 2L three-necked flask, add 240ml of 2mol / L hydrochloric acid dropwise under an ice bath; stir to dissolve; separate the liquids, collect the organic layer, and wash it twice with 600ml of water; Under reduced pressure at 38°C, the free acid of AHU377 was obtained;
[0079] Preparation of LCZ696:
[0080] At room temperature, add AHU377 free acid, 100g valsartan, 880ml acetone, and 220ml isopropanol to a 3L three-necked flask and dissolve; add dropwise an aqueous solution of sodium hydroxide with an equivalent concentration of 0.9g / ml relative to valsartan 2.85 , heat up to 50°C and react for 20 minutes; cool down to 45°C, add about 2.0 g of seed crystals, the solution becomes cloudy after stirring, then lower the external temperature to 35°C at a rate of 1.0°C / 10min, and then move the reaction solution to 15°C Stir at high temperature for 2h; then add 1120m...
Embodiment 2
[0083] Preparation of AHU377 free acid:
[0084] Add 100g of AHU377 calcium salt and 1000ml of isopropyl acetate into a 2L three-neck flask, add 230ml of 2mol / L hydrochloric acid dropwise under ice bath; stir to dissolve; separate the liquid, collect the organic layer, and wash it twice with 600ml of water; Desolvation under reduced pressure at 38°C to obtain AHU377 free acid, add 250ml of isopropanol to dissolve, then desolventize under reduced pressure again and repeat once;
[0085] Preparation of LCZ696:
[0086] At room temperature, add AHU377 free acid, 100g valsartan, 800ml acetone, and 220ml isopropanol to a 3L three-necked flask and dissolve; add dropwise at room temperature an aqueous solution of sodium hydroxide with an equivalent concentration of 0.85g / ml relative to valsartan 2.95 , raised the temperature to 53°C for 30 minutes; then lowered the external temperature to 30°C at a rate of 1.0°C / 10min, added about 1.0 g of seed crystals, moved the reaction solution ...
Embodiment 3
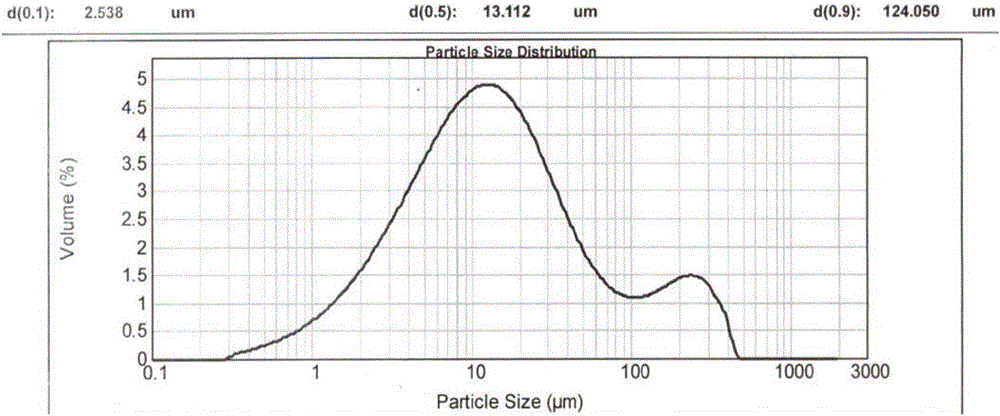
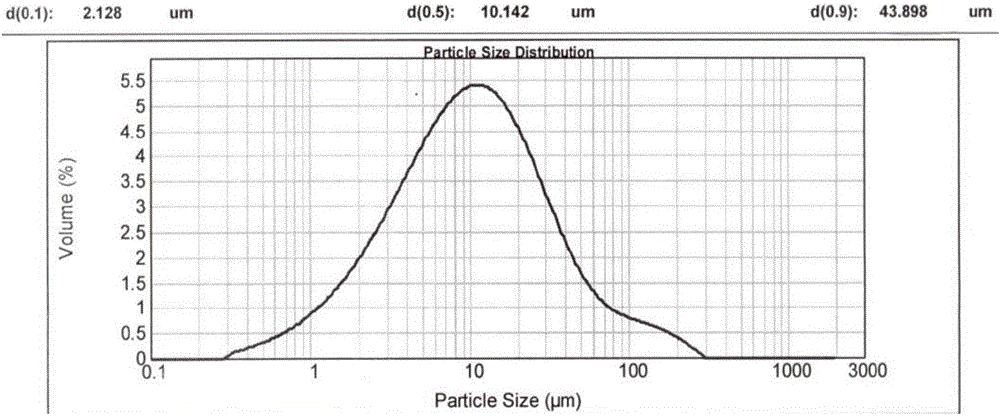
[0089] Since the crystalline powder obtained in Examples 1 and 2 has a moderate particle size and shape, rapid filtration can be achieved during the reaction. In order to reflect its advantages in filtration compared with the prior art, the reaction scale of embodiment 1 and embodiment 2 is enlarged by 20 times, and it is compared with patent CN200680001733.0 embodiment 3 (hereinafter referred to as "comparative implementation Example 1") for comparison, the results are as follows:
[0090] project Suction time (min) D90(μm) Example 1 about 20min 44.68 Example 2 <20min
47.36 Comparative Example 1 about 55min 16.80
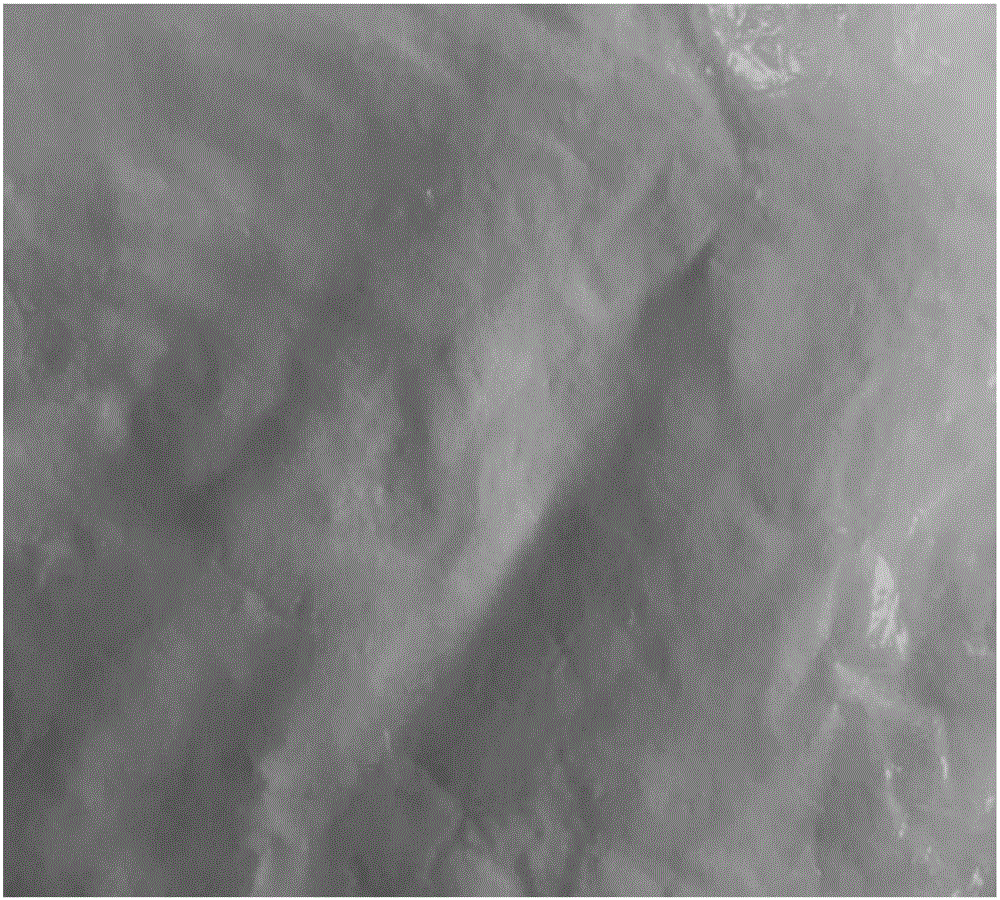
[0091] In particular, the crystalline powder obtained in Comparative Example 1 has a more serious caking phenomenon (appearance such as Figure 6 shown), showing that the passing rate through the 80 mesh sieve is only about 25%, and the particle size distribution of the resulting crystalline powder is as follows Figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com