Method for synthesizing 3-fluoro-4-(4-morpholinyl)aniline by using micro-channel reactor
A micro-channel reactor, morpholine-based technology, applied in organic chemistry and other directions, can solve problems such as polynitration, complex process operation, and oxidation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
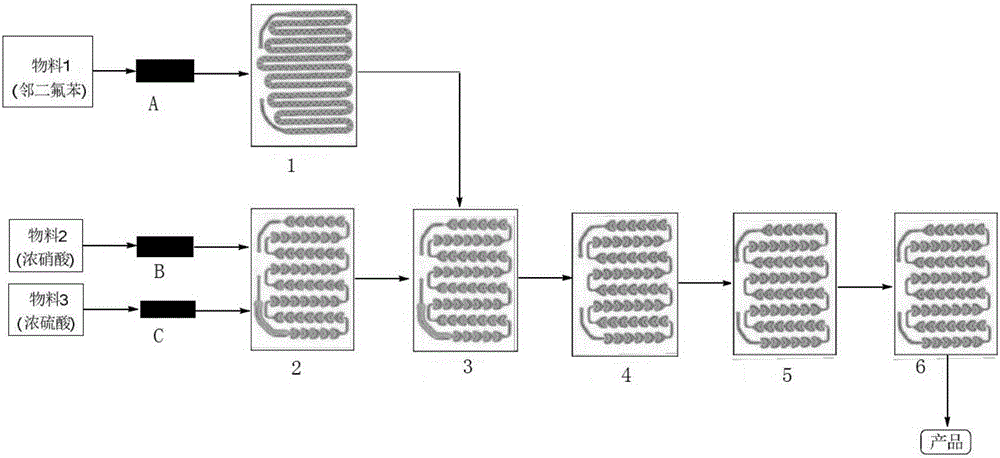
[0076] (1) Weigh 300g of o-difluorobenzene as material 1, measure 400ml of concentrated nitric acid as material 2, and measure 600mL of concentrated sulfuric acid as material 3;
[0077] (2) The flow velocity of control material 1 is 8ml / min; The flow velocity of control material 2 is 12min / min; The flow velocity of control material 3 is 18min / min; Reaction temperature is 20 ℃, and the mol ratio of o-difluorobenzene and nitric acid is 1 :2.5; The mol ratio of nitric acid and the vitriol oil is 1:2.0; The residence time of reaction is 65s;
[0078](3) After each strand of material in the reactor reaches a steady state, collect the reaction solution flowing out from the reactor outlet, and feed the corresponding reaction solution of material 1 (that is, 240ml of material 1, 275.0g of o-difluorobenzene) for 30 minutes For example, separate the liquid, keep the organic phase, extract the acid layer once with 300ml of dichloromethane, combine the organic phases, add 300ml of 10% Na...
Embodiment 2
[0086] In the screening and optimization process of process parameters, we explored the parameters such as the concentration of the reaction raw materials, molar ratio, residence time, etc., and changed the corresponding parameters of the corresponding steps on the basis of the operation steps in Example 1, and kept other parameters unchanged. , the results are summarized as follows:
[0087] 1. Nitrification reaction:
[0088]
[0089] 2. Nucleophilic substitution reaction:
[0090]
[0091] 3. Catalytic hydrogenation reaction:
[0092]
[0093]
[0094] 4. Pd / C recovery application experiment:
[0095]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com