Method for purifying dabigatran etexilate mesylate intermediate
A technology of dabigatran etexilate mesylate and purification method, which is applied in the field of purification of dabigatran etexilate mesylate intermediate, and can solve problems such as failure to meet quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Dabigatran etexilate mesylate intermediate (N-[[2-[[[(4-(aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-5- Preparation of benzimidazole]carbonyl]-N-2-pyridyl-β-alanine ethyl ester p-toluenesulfonate)
[0049] Reference WO2012152855 Example 1, g step operation to prepare dabigatran etexilate mesylate intermediate.
[0050] To a 1000mL glass reaction flask, add 47.7g of compound C (3-[[[2-[[(4-cyanophenyl)amino]methyl]-1-methyl-H-benzimidazole-5 -yl] carbonyl] pyridin-2-yl amino] ethyl propionate oxalate), 21.8g p-toluenesulfonic acid and 142g of 10mol / L hydrogen chloride ethanol solution. The reaction solution was incubated at room temperature for 24 hours, 400 mL of ethanol was added, the temperature was lowered to 0°C, ammonia gas was slowly introduced at 0°C until the precipitation was complete, and then the temperature was slowly raised to 10°C for 2 hours, and the temperature was raised to room temperature for overnight. The solvent was distilled off under redu...
Embodiment 2
[0052] Dabigatran etexilate mesylate intermediate (N-[[2-[[[(4-(aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-5- Purification of benzimidazole]carbonyl]-N-2-pyridyl-β-alanine ethyl ester p-toluenesulfonate)
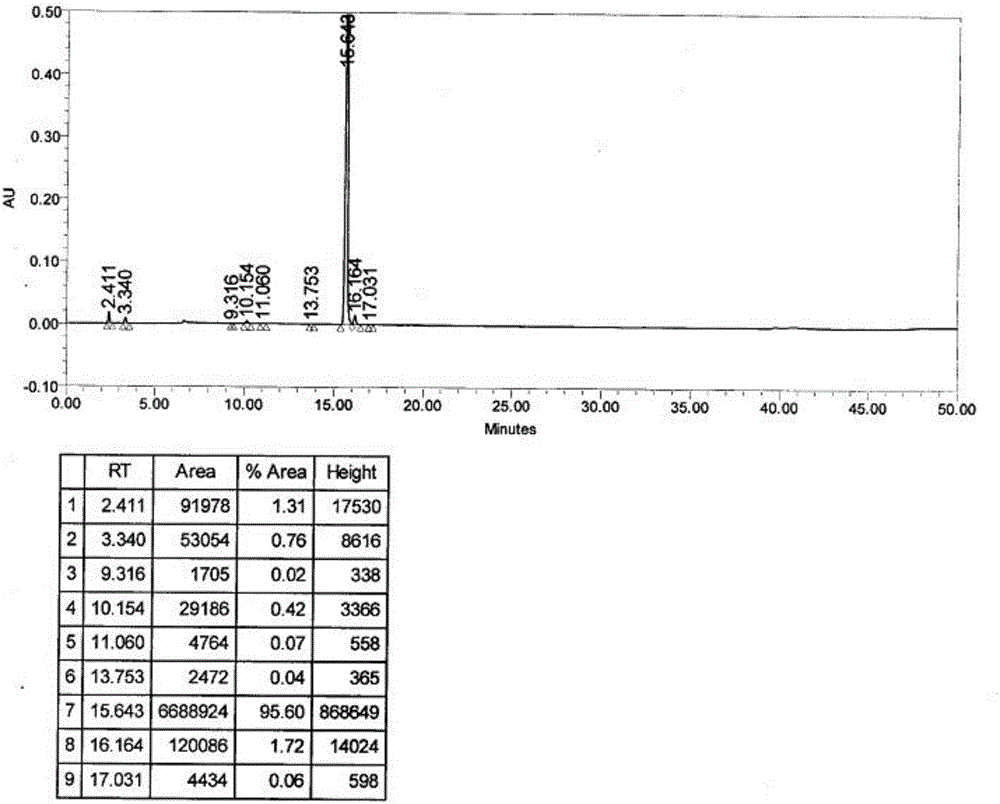
[0053]Add 34.0 g of the intermediate prepared in Example 1 into a 1000 mL glass reaction bottle, add 150 mL of ethanol and 200 mL of water in sequence, heat the reaction liquid in a water bath to 80-100° C. and keep it warm for 30 minutes, then heat filter and discard the filter cake. Transfer the filtrate into a 1000mL glass reaction bottle, raise the temperature until all the solids are dissolved, and perform gradient crystallization operation: control the rotation speed at 20-30 rpm, control the cooling rate at 5°C / min, and cool down to the time when solids start to precipitate (initial crystallization) Stop cooling and stir for 20 minutes; control the cooling rate to 5°C / min, add 200 mL of water dropwise at a rate of 5 mL / min, stop cooling at the same time when th...
Embodiment 3
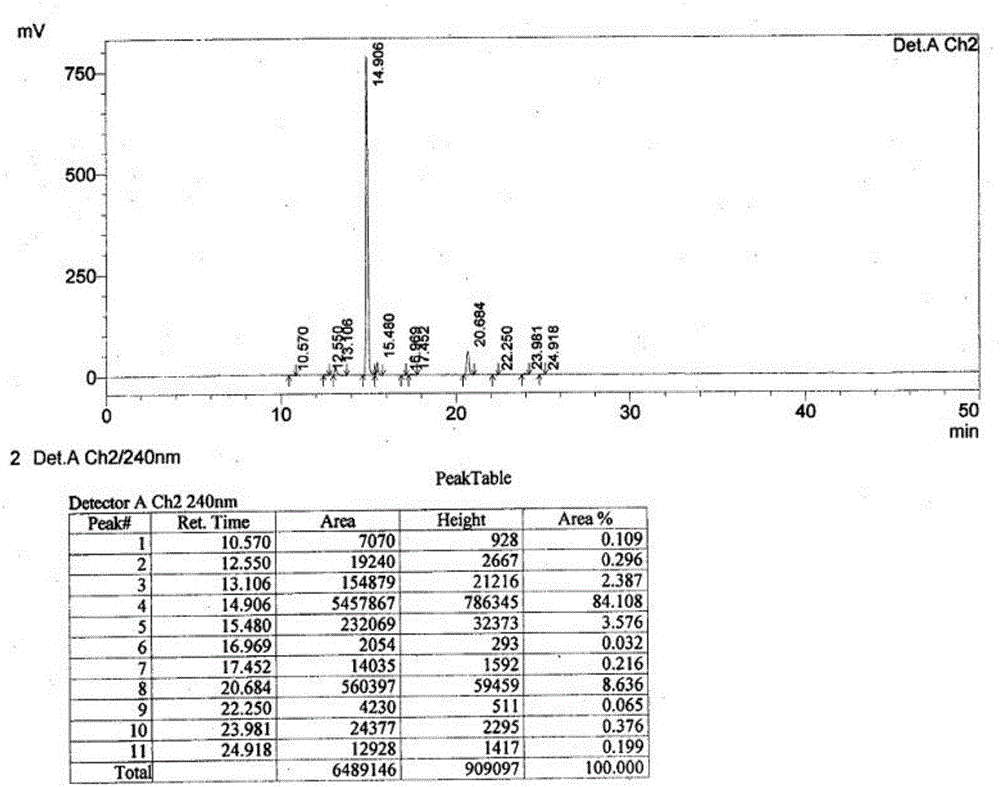
[0056] Dabigatran etexilate mesylate intermediate (N-[[2-[[[(4-(ammonia results as attached figure 2 As shown, using analytical method 1, area normalized method: iminomethyl)phenyl]amino]methyl]-1-methyl-1H-5-benzimidazole]carbonyl]-N-2-pyridyl -Purification of β-alanine ethyl ester p-toluenesulfonate)
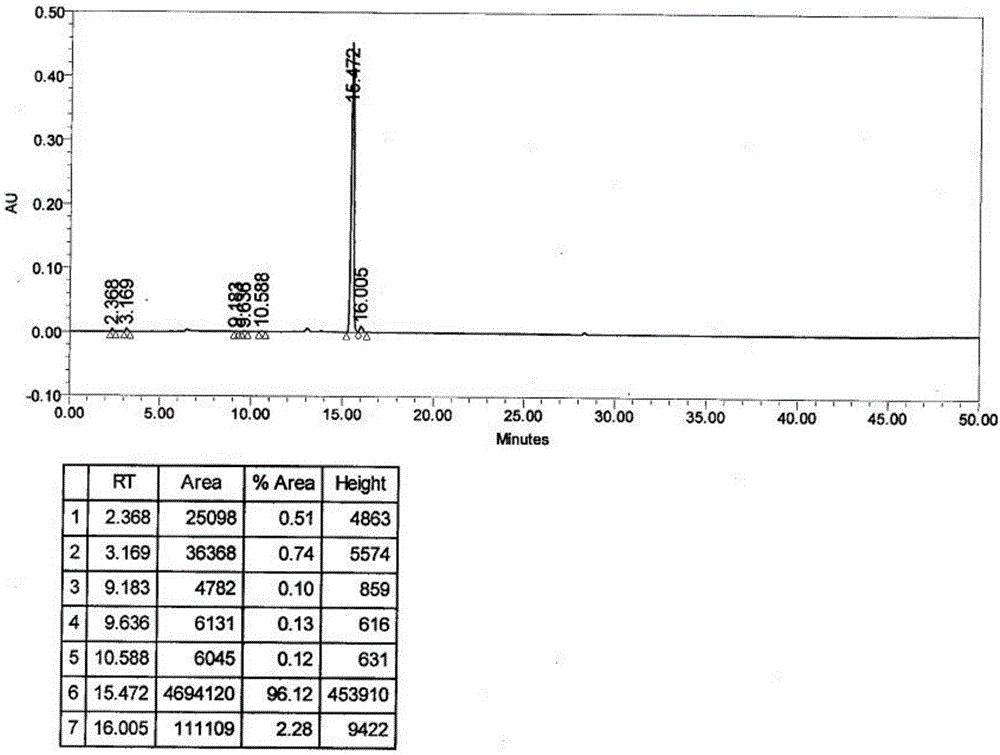
[0057] Add 34.0 g of the intermediate prepared in Example 1 into a 1000 mL glass reaction bottle, add 150 mL of methanol and 150 mL of water in sequence, heat the reaction liquid in a water bath to 75-100 ° C for 30 minutes, heat filter, and discard the filter cake. Transfer the filtrate into a 1000mL glass reaction bottle, raise the temperature until all the solids are dissolved, and perform gradient crystallization operation: control the rotation speed at 20-30 rpm, control the cooling rate at 5°C / min, and cool down to the time when solids start to precipitate (initial crystallization) Stop cooling and stir for 20 minutes; control the cooling rate to 5°C / min, add 200 mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com