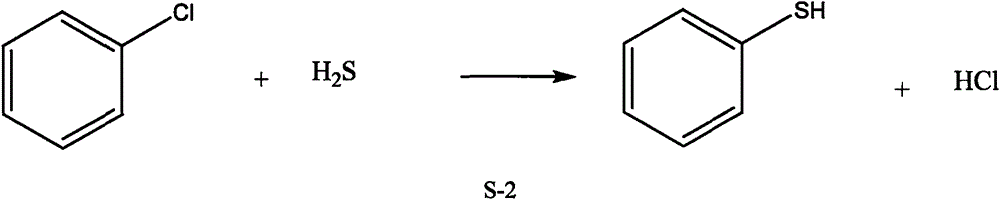
Supercritical phenthiol synthesis method by using hydrogen sulfide
A hydrogen sulfide, supercritical technology, applied in the direction of mercaptan preparation, organic chemistry, bulk chemical production, etc., can solve the problems of high reaction temperature, high corrosiveness, high mercaptan price, etc., achieve simple reaction raw materials and reduce process costs , enhance the effect of reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: a kind of synthetic method of thiophenol, carries out following steps successively:
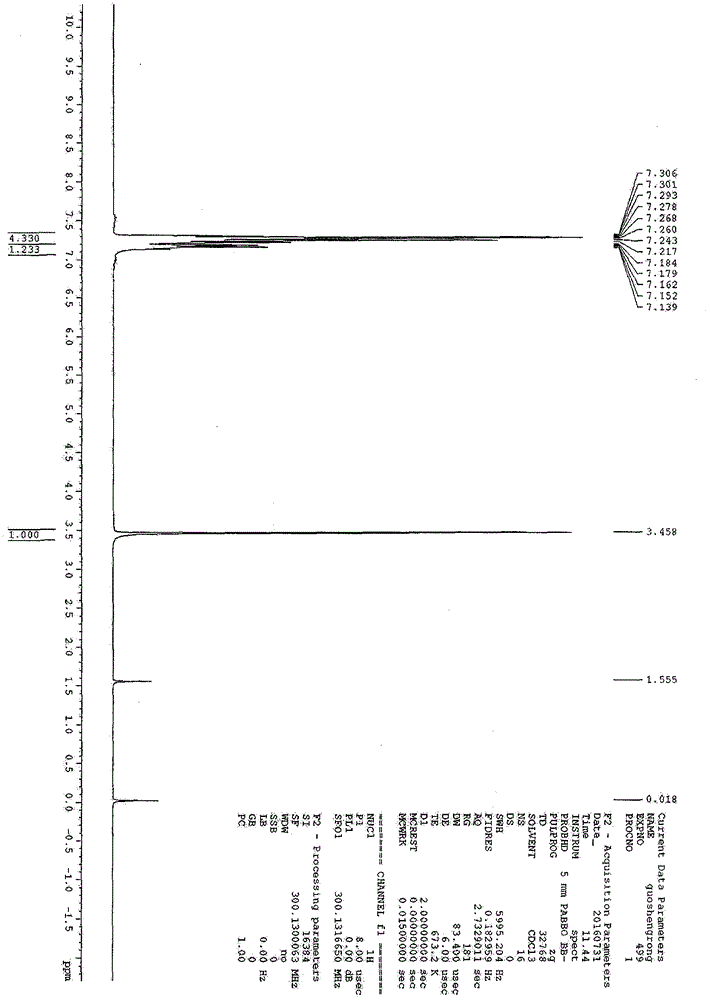
[0025] Add 112.5g (1.0mol) of chlorobenzene to a 250ml titanium autoclave (with mechanical stirring, temperature measuring probe, electric heating mantle, cooling coil, bottom pipe, vent valve and safety valve), and replace with nitrogen three times Vent, then press 68g (2.0mol) of hydrogen sulfide into the bottom tube, heat to 110°C, and the pressure at this time is 10.5MPa. After keeping this temperature for 7 hours, finish the reaction, cool to room temperature, release hydrogen chloride and excess hydrogen sulfide (alkali absorption) through the vent valve, and return to normal pressure of the reaction system. Open the autoclave, wash the reaction mixture with 200mL*3 deionized water, combine and collect the oil phases washed three times for direct vacuum distillation, and collect (10mmHg, 4749°C) fraction 103.2g, which is the product thiophenol, with a yield of 93.8 ...
Embodiment 2
[0026] Embodiment 2: a kind of synthetic method of thiophenol, carries out following steps successively:
[0027] Add 56.3g (0.5mol) of chlorobenzene to a 250ml titanium autoclave (with mechanical stirring, temperature measuring probe, electric heating mantle, cooling coil, bottom pipe, vent valve and safety valve), and replace with nitrogen three times Empty, then press 51g (1.5mol) of hydrogen sulfide from the dip tube, heat to 125°C, and the pressure at this time is 11.5MPa. After maintaining the temperature for 3 hours, the reaction was terminated, cooled to room temperature, hydrogen chloride and excess hydrogen sulfide (alkali absorption) were released through the vent valve, and the reaction system was brought to normal pressure. Open the autoclave, wash the reaction mixture with 200mL*3 deionized water, combine and collect the oil phases washed three times for direct vacuum distillation, and collect (10mmHg, 47-49°C) fraction 50.8g, which is the product thiophenol. Th...
Embodiment 3
[0028] Embodiment 3: a kind of synthetic method of thiophenol, carries out following steps successively:
[0029] Add 28.1g (0.25mol) of chlorobenzene to a 250ml titanium autoclave (with mechanical stirring, temperature measuring probe, electric heating mantle, cooling coil, bottom pipe, vent valve and safety valve), and replace with nitrogen three times Empty, then press 42.5g (1.25mol) of hydrogen sulfide from the dip tube, heat to 140°C, and the pressure at this time is 13.3MPa. After keeping this temperature for 5 hours, finish the reaction, cool to room temperature, release hydrogen chloride and excess hydrogen sulfide (alkali absorption) through the vent valve, and return to normal pressure of the reaction system. Open the autoclave, wash the reaction mixture with 200mL*3 deionized water, combine and collect the oil phases washed three times for direct vacuum distillation, and collect (10mmHg, 47-49°C) fraction 26.0g, which is the product thiophenol. The yield was 94.6%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com