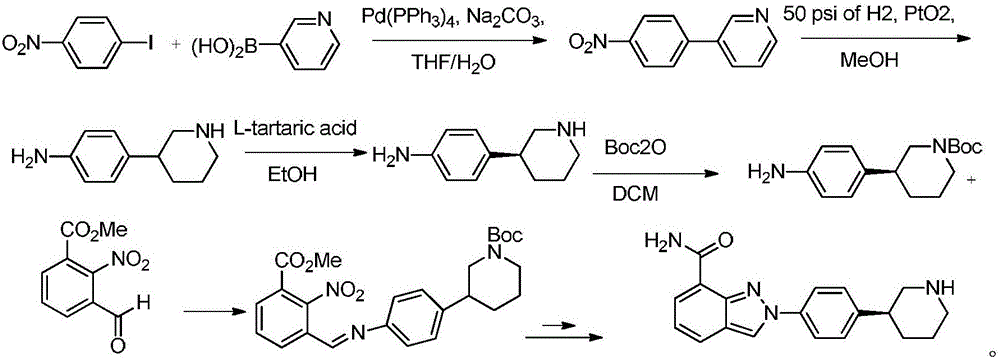
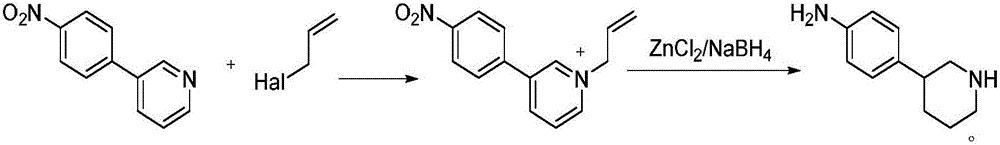
Method for preparing 4-(piperidine-3-yl)aniline
A technology of piperidine and aniline, which is applied in the field of preparation of 4-aniline, an intermediate of anticancer drug nirapabib, can solve the problems of low cost, difficult purification of by-products, and high yield, and achieves reduced production cost and good yield. , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] N-allyl-3-(4-nitrophenyl)pyridine quaternary ammonium salt
[0025] 20g (100mmol) of 3-(4-nitrophenyl)pyridine and 1.3g (20mmol) of zinc powder were added to a reaction vessel containing 100ml of acetonitrile, then 13.3g (110mmol) of 3-bromopropene was added at 65°C, React for 2 hours, filter, wash the filter cake three times with acetonitrile, combine the filtrate washings, concentrate, then recrystallize from petroleum ether, and dry in vacuo to obtain N-allyl-3-(4-nitrophenyl)pyridinium quaternary ammonium salt 31.4 g, yield 97.7%. HRMS (ESI + )m / z Calculated C 18 h 16 N 2 o 2 (M-Br) + 242.1037, Found 242.1045.
Embodiment 2
[0027] N-allyl-3-(4-nitrophenyl)pyridine quaternary ammonium salt
[0028] 20g (100mmol) of 3-(4-nitrophenyl)pyridine and 1.95g (30mmol) of zinc powder were added to a reaction vessel containing 100ml of acetonitrile, then 13.3g (110mmol) of 3-bromopropene was added at 55°C, React for 2.5 hours, filter, wash the filter cake three times with acetonitrile, combine the filtrate and filter cake washing with acetonitrile, concentrate in vacuo, then recrystallize from petroleum ether, and dry in vacuo to obtain N-allyl-3-(4-nitrophenyl) Pyridinium quaternary ammonium salt 31.6g, yield 98.4%.
Embodiment 3
[0030] N-allyl-3-(4-nitrophenyl)pyridine quaternary ammonium salt
[0031] 20g (100mmol) of 3-(4-nitrophenyl)pyridine and 0.65g (10mmol) of zinc powder were added to a reaction vessel containing 100ml of acetonitrile, then 14.5g (120mmol) of 3-bromopropene was added at 60°C, React for 2 hours, filter, wash the filter cake three times with acetonitrile, combine the filtrate and filter cake washing with acetonitrile, concentrate in vacuo, then recrystallize from petroleum ether, and dry in vacuo to obtain N-allyl-3-(4-nitrophenyl) Pyridinium quaternary ammonium salt 31.3g, yield 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com