Droplet PCR (Polymerase Chain Reaction) kit for detecting mutation of human KRAS gene
A kit and human detection technology, applied in the direction of DNA/RNA fragments, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problems of difficult acquisition of tissue samples, and achieve dynamic tracking, convenient and accurate analysis of experimental data The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
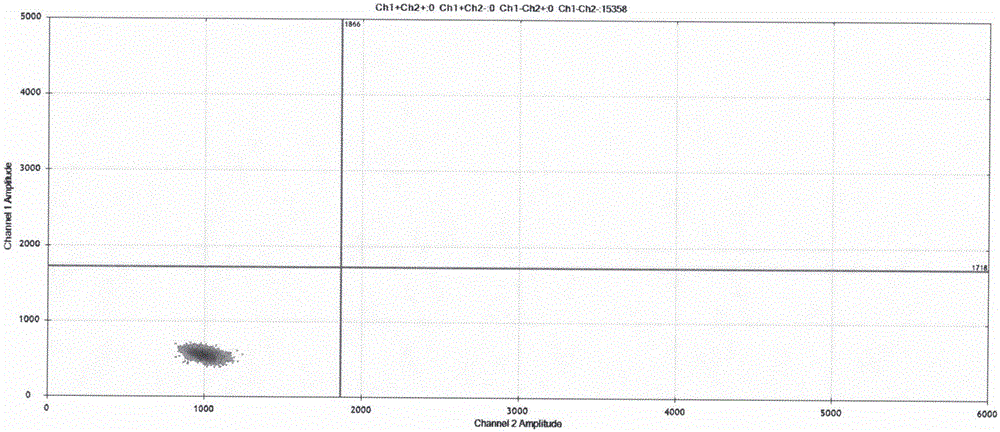
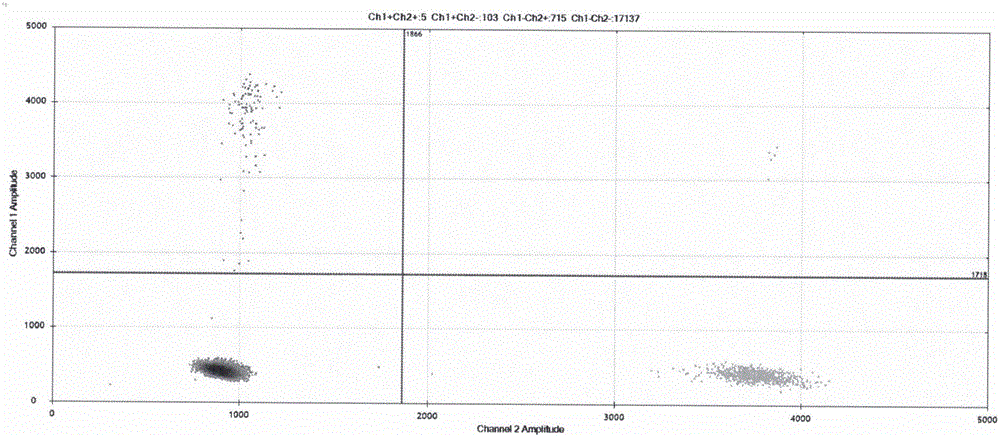
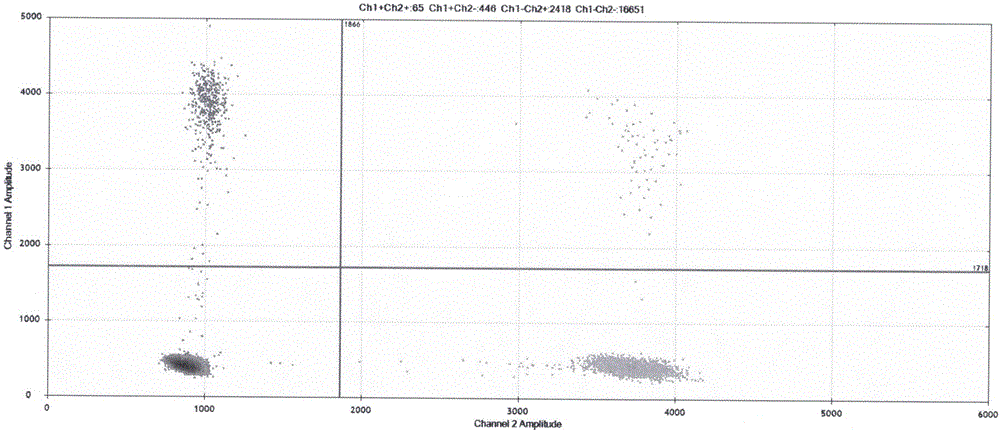
Image
Examples
Embodiment 1
[0095] A microdroplet PCR kit for detecting human KRAS gene mutation, its composition is as shown in Table 7:
[0096] Table 7:
[0097]
[0098] The packaging and content of each component of the droplet PCR kit in this embodiment are as shown in Table 8:
[0099] Table 8:
[0100]
[0101]
Embodiment 2
[0102] Example 2: Sample linearity detection of the kit of the present application
[0103] 1. Prepare linear quality control products
[0104] Select the K-ras gene G12D mutant plasmid DNA (SEQ ID NO: 12) and K-ras negative HT-1080 cell line genomic DNA to mix in proportion, the total genomic DNA concentration is 5000 copies / μl, and the mutation ratio is 27%. The above samples As the sample linear quality control product L1 of the kit of Example 1 of the present application, the L1 sample was serially diluted with 5000 copies / μl of K-ras-negative HT-1080 cell line genomic DNA in a 3-fold gradient to obtain the linear properties respectively. Control products L2, L3 and L4 were made into linear quality control products. Specifically as shown in Table 9:
[0105] Table 9:
[0106]
[0107] 2. Amplification reagent preparation: Take out the KRAS(exon18)ddPCR reaction solution from the kit, melt and mix at room temperature, centrifuge at 2000rpm for 10s, and prepare the PCR...
Embodiment 3
[0125] Example 3: Detection of the accuracy of the kit of the present application
[0126] 1. Preparation of accurate quality control products
[0127] Select qualified K-ras positive cell line genomic DNA and mutated plasmid DNA, mix with K-ras negative HT-1080 cell line genomic DNA in proportion to form the accurate quality control product of the kit of Example 1 of the present application. Specifically as shown in Table 11:
[0128] Table 11:
[0129]
[0130]
[0131] 2, other steps are with embodiment 2;
[0132] The obtained experimental results are shown in Table 12:
[0133] Table 12:
[0134]
[0135] From the experimental results in Table 12, it can be seen that the positive coincidence rate of the test results is 100% for each accurate quality control product in the corresponding reaction solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com