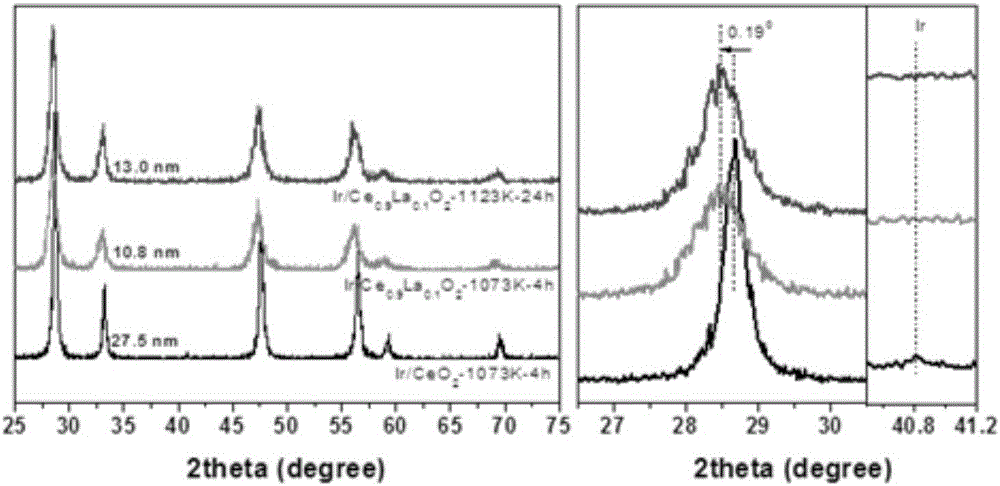
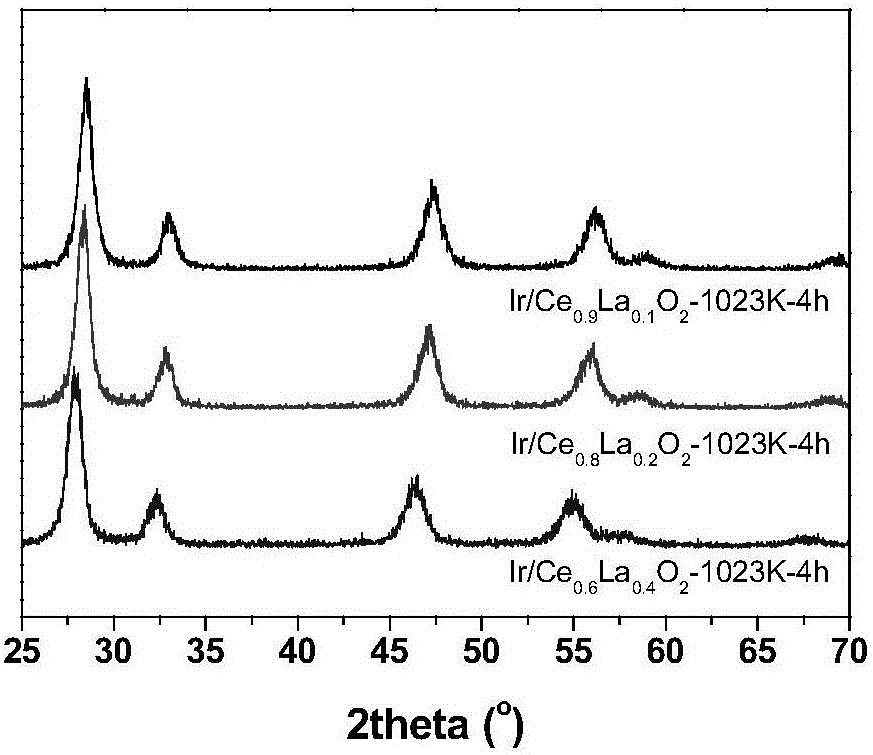
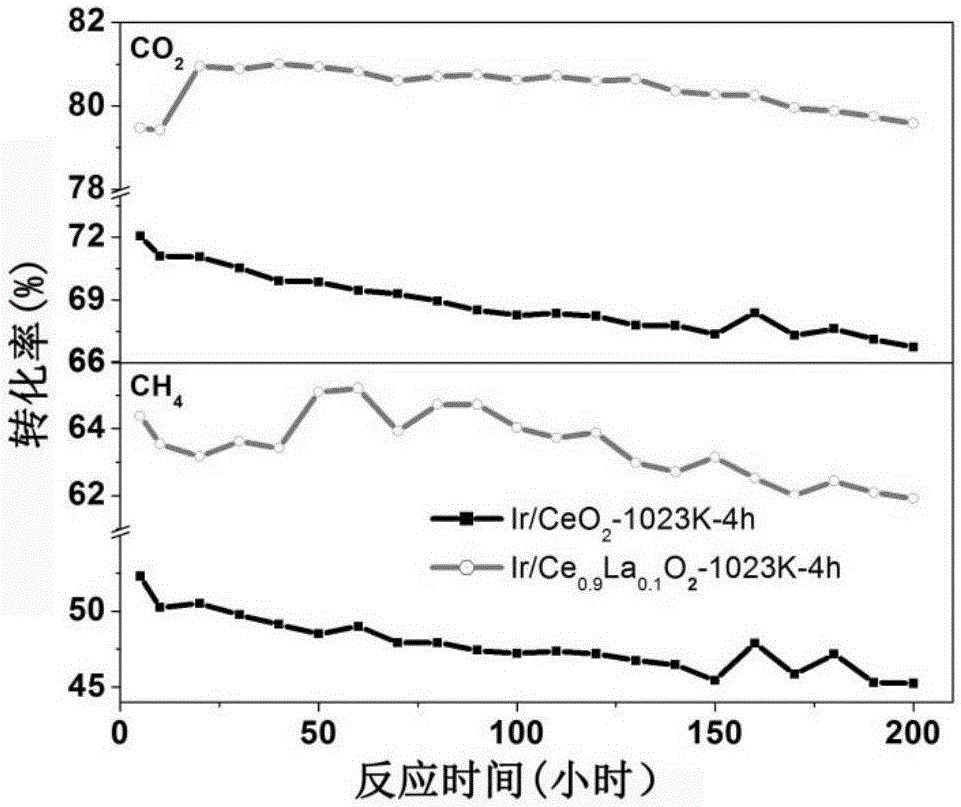
Cerium oxide-based precious metal nanometer catalyst and preparation method thereof
A nano-catalyst, cerium oxide-based technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. It is prone to problems such as sintering and structural instability, so as to achieve the effect of stable catalytic material structure, simple preparation process and improved structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] [Example 1] Preparation of Ir / CeO 2 -1023K-4h and Ir / CeO 2 -1073K-4h catalyst
[0039] This embodiment is Ir / CeO 2 -1023K-4h and Ir / CeO 2 -The embodiment of the preparation method of 1073K-4h catalyst, comprises following preparation steps:
[0040] a. Dissolving an appropriate amount of cerium nitrate (final concentration of 0.03-0.06mol / L) in 200mL of deionized water, mixed uniformly to obtain a mixed solution of cerium nitrate;
[0041] b. Dissolving urea (2mol / L-4mol / L) in 200mL deionized water until completely dissolved to obtain an aqueous urea solution;
[0042] c. the cerium nitrate solution obtained in step a is quickly poured into the aqueous urea solution obtained in step b, and fully stirred for 30 minutes to obtain the mixture;
[0043] d. Heat the mixture obtained in step c to 90°C in a water bath and keep the temperature constant for 4-5 hours to obtain a suspension;
[0044] e. filter and wash the suspension obtained in step d, then dry at 100°C fo...
Embodiment 2
[0049] [Example 2] Preparation of Ir / Ce 0.9 La 0.1 o 2 -1073K-4h catalyst
[0050] This embodiment is Ir / Ce 0.9 La 0.1 o 2 -The embodiment of the preparation method of 1073K-4h catalyst, comprises following preparation steps:
[0051] a. an appropriate amount of cerium nitrate (0.06mol / L) and lanthanum nitrate (0.0067mol / L) are dissolved in 200mL deionized water, mix well, wherein the mol ratio of cerium nitrate and lanthanum nitrate is 9:1, obtains cerium nitrate and a mixed solution of lanthanum nitrate;
[0052] b. Dissolving urea (2mol / L-4mol / L) in 200mL deionized water until completely dissolved to obtain an aqueous urea solution;
[0053] c. The mixed solution of cerium nitrate and lanthanum nitrate obtained in step a is quickly poured into the aqueous urea solution obtained in step b, and fully stirred for 30 minutes to obtain the mixture;
[0054] d. Heat the mixture obtained in step c to 90°C in a water bath and keep the temperature constant for 4-5 hours to o...
Embodiment 3
[0060] [Example 3] Preparation of Ir / Ce 0.9 La 0.1 o 2 -1123K-4h catalyst
[0061] This embodiment is Ir / Ce 0.9 La 0.1 o 2 -The embodiment of the preparation method of 1123K-4h catalyst, comprises following preparation steps:
[0062] a. Dissolve an appropriate amount of cerium nitrate (0.06mol / L) and lanthanum nitrate (0.0067mol / L) in 200mL deionized water, the molar ratio of cerium nitrate and lanthanum nitrate is 9:1, mix well to obtain cerium nitrate and lanthanum nitrate mixed solution;
[0063] b. Dissolving urea (2mol / L-4mol / L) in 200mL deionized water until completely dissolved to obtain an aqueous urea solution;
[0064] c. Pour the mixed solution of cerium nitrate and lanthanum nitrate obtained in step a into the aqueous urea solution obtained in step b quickly, and fully stir to obtain the mixture for 30 minutes;
[0065] d. Heat the mixture obtained in step c to 90°C in a water bath and keep the temperature constant for 4-5 hours to obtain a suspension;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com