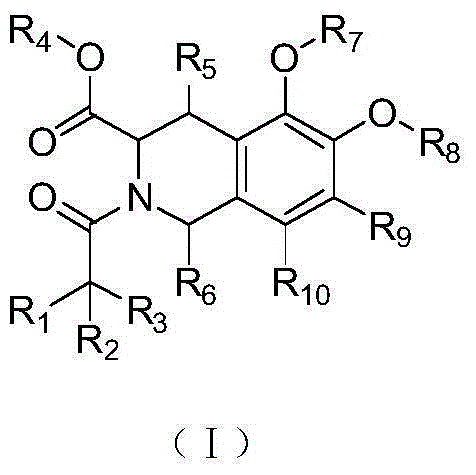
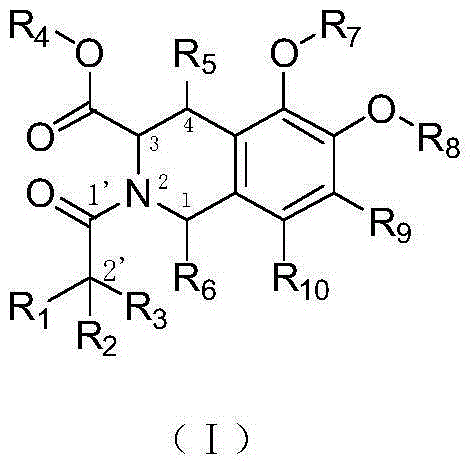
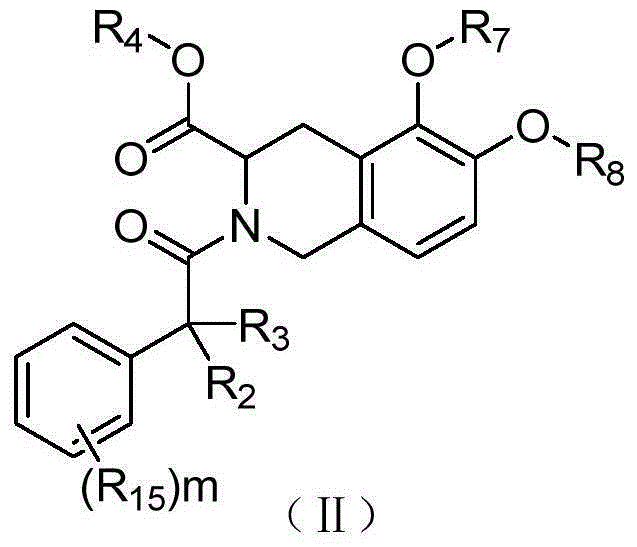
1,2,3,4-Tetrahydroisoquinoline derivatives, and preparation method and application thereof
A technology of tetrahydroisoquinoline and its derivatives, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., and can solve the problems of drug resistance and dependence, cognitive changes, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] Preparation of intermediates
[0117] 1. Intermediate 1: Preparation of ethyl 3-(2-(benzyloxy)-3-methoxyphenyl)-2-((diphenylmethylene)amino)propionate
[0118]
[0119] To a solution of 2-(benzyloxy)-1-(chloromethyl)-3-methoxybenzene (6.8 g, 25.88 mmol) in acetonitrile (60 mL) was added potassium iodide (5.59 g, 33.65 mmol) at room temperature , cesium carbonate (16.87g, 51.76mmol), ethyl 2-((diphenylmethylene)amino)acetate (6.92g, 25.88mmol), then stirred overnight in a 50°C oil bath under nitrogen protection . After cooling, the organic solvent was removed by rotary evaporation under reduced pressure, ethyl acetate and a water layer were added, the ethyl acetate phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated for column chromatography (eluent: petroleum ether / ethyl acetate=10:1) to give ethyl 3-(2-(benzyloxy)-3-methoxyphenyl)-2-((diphenylmethylene)amino)propionate (8.9g, 70%).
[0120] MS m / z(ESI): 494.6[M+H] + ....
Embodiment 1
[0130] Example 1: 5-(benzyloxy)-6-methoxy-2-(1-phenylcyclohexane-1-carbonyl)-1,2,3,4-tetrahydroisoquinoline-3- Preparation of Carboxylic Acids
[0131]
[0132] The first step: the preparation of 1-phenylcyclohexane-1-carbonyl acid chloride
[0133]
[0134] At room temperature, oxalyl chloride (248 mg, 1.96 mmol) and DMF (0.2 mL) were added to a solution of 1-phenylcyclohexane-1-carboxylic acid (200 mg, 0.98 mmol) in dichloromethane (5 mL), and the reaction was carried out at room temperature. After stirring for 2 hours under reduced pressure, the solvent was removed under reduced pressure to obtain crude 1-phenylcyclohexane-1-carbonyl acid chloride (230 mg), which was directly used in the next reaction.
[0135] Step 2: Ethyl 5-(benzyloxy)-6-methoxy-2-(1-phenylcyclohexane-1-carbonyl)-1,2,3,4-tetrahydroisoquinoline- Preparation of 3-carboxylate
[0136]
[0137] To a solution of 1-phenylcyclohexane-1-carbonyl acid chloride (100 mg, 0.45 mmol) in dichloromethane (5...
Embodiment 2
[0143] Example 2: 5-(benzyloxy)-6-methoxy-2-(1-phenylcyclopropane-1-carbonyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxy Preparation of acid
[0144]
[0145] Preparation method of 5-(benzyloxy)-6-methoxy-2-(1-phenylcyclopropane-1-carbonyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid Refer to Example 1. MS m / z(ESI): 458.2[M+H] + ;
[0146] 1 H NMR (400MHz, CDCl 3 )δ7.41-7.28(m,6H),7.23-7.13(m,4H),6.88-6.69(m,1H),6.68-6.44(m,1H),5.11-4.82(m,3H),4.66- 4.13 (m, 2H), 3.84 (s, 3H), 3.40-2.75 (m, 2H), 1.54-1.19 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com