Colorimetric probe based on naphthalimides derivative and preparation method and application thereof
A technology of hydrazinonaphthalimide and naphthalimide, which is applied in the field of naphthalimide derivatives and its preparation, can solve problems such as decreased arterial elasticity, elevated cholesterol, and elevated blood pressure, achieving strong selectivity and sensitivity High and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of Naphthalimide Derivatives
[0025]
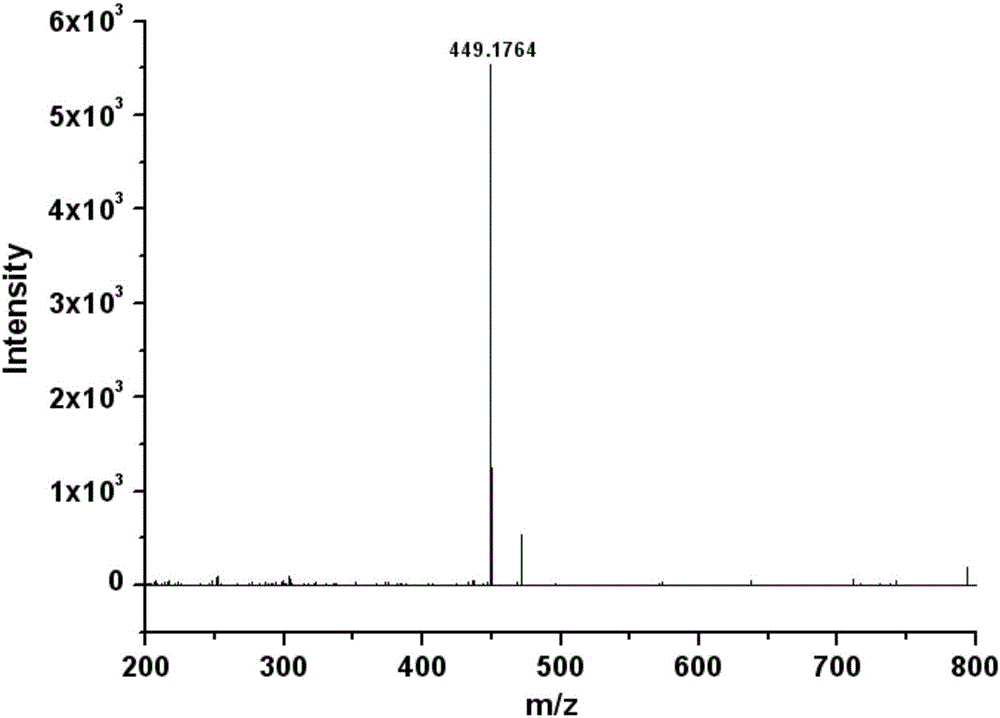
[0026] Dissolve 0.195 g of ethyl 5-formyl-2,4-dimethylpyrrole-3-carboxylate in 10 mL of absolute ethanol, then add 0.271 g of N-hydroxyethyl-4-hydrazinonaphthalimide, usually Stir under pressure at 78°C for 3 h, cool to room temperature and precipitate a large amount of solid, filter under reduced pressure, recrystallize the filter residue with acetonitrile to obtain a red solid which is the target product, and the yield of the target product is 78.2%.
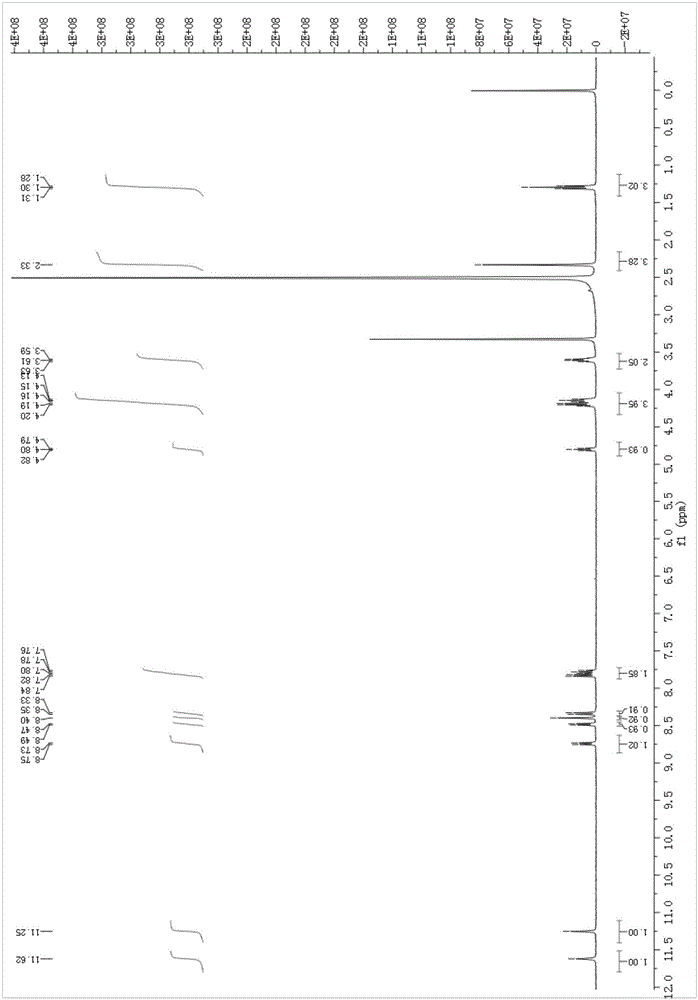
[0027] Naphthalimide derivative NMR analysis results are as follows:
[0028] 1 H NMR (400 MHz, DMSO- d 6 ), δ (ppm): 11.62 (s, 1H, OH), 11.25 (s, 1H, NH),8.73-8.75 (d, 1H, Aryl-H), 8.47-8.49 (d, 1H, Aryl-H) , 8.40 (s, 1H, CH=N),8.33-8.35 (d, 1H, Aryl-H), 7.76-7.84 (m, 2H, Aryl-H), 4.79-4.82 (t, 1H, OH),4.13 -4.20 (m, 4H, 2CH 2 ), 3.59-3.63 (q, 2H, CH 2 ), 2.33 (s, 3H, CH 3 ), 1.29-1.31(t, 3H, CH 3 ).
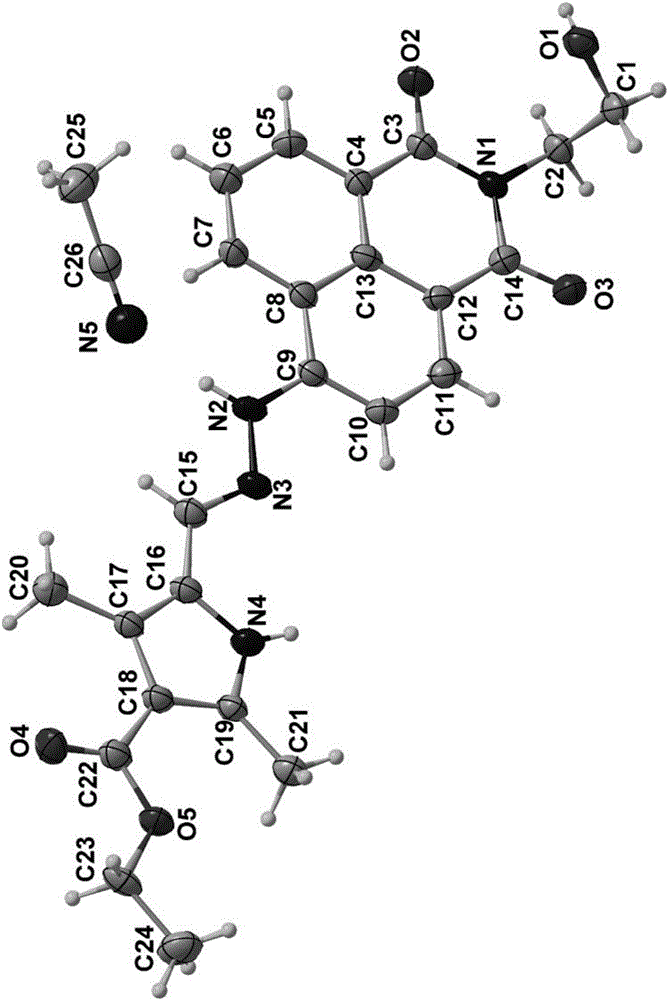
[0029] Single crystal structure: see the crystal struct...
Embodiment 2
[0032] The preparation method of the colorimetric probe based on naphthalimide derivatives: 0.001 mol of 5-formyl-2,4-dimethylpyrrole-3-carboxylic acid ethyl ester was dissolved in 50ml of absolute ethanol, Then add 0.01 mol of N-hydroxyethyl-4-hydrazinonaphthalimide, reflux and stir at 70°C under normal pressure for 1h, cool to room temperature and precipitate a solid, filter under reduced pressure, and recrystallize the filter residue with acetonitrile to obtain a naphthalene-based Colorimetric probes for imine derivatives.
Embodiment 3
[0034] The preparation method of the colorimetric probe based on naphthalimide derivatives: 0.01mol of 5-formyl-2,4-dimethylpyrrole-3-carboxylic acid ethyl ester is dissolved in 10 ml of absolute ethanol , then add 0.001 of N-hydroxyethyl-4-hydrazinonaphthalimide, stir at 90°C under normal pressure for 3h, cool to room temperature and precipitate a solid, filter under reduced pressure, and recrystallize the filter residue with acetonitrile to obtain Naphthaloyl-based Colorimetric probes for imine derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com