Fluorescent Probes for Analytical Detection and Screening of Galactokinase Inhibitors
A technology of galactokinase and fluorescent probe, which is applied in the field of biological analysis and detection, can solve the problems of test result interference, tedious preparation and purification of galactokinase, and achieve the effect of simplifying the screening process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Compound (I-1) is synthesized in the following manner
[0059]
[0060] (1) Synthesis of compound 1a:
[0061] 1) Add cy3 (246.5mg) to dry DMF (dimethylformamide) (10ml) at room temperature, cool to 0°C, replace the air in the flask with nitrogen, and add TBTU(O- Benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroborate) (176.5 mg).
[0062] 2) Stir the reaction solution at room temperature for 15 minutes under the protection of nitrogen, then sequentially add 6-amino-6-deoxy-1,2,3,4-di-oxo-isopropyl-α-D-galactose (142.62mg ) and triethylamine (TEA) (151.8 mg), the reaction solution was reacted at room temperature for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the organic phase was extracted with dichloromethane solution to obtain a crude product. The reaction product was simply purified by silica gel column chromatography (dichloromethane:methanol=45:1) to obtain 304.7 mg of a blue solid product .
[0063] Y...
Embodiment 2
[0070] Compound (I-2) is synthesized in the following manner
[0071]
[0072] (1) Synthesis of compound 2a:
[0073] 1) Add cy3 (200.5 mg) and 1,2:3,4-oxo-isopropylidene-α-D-galactofuranose (106.0 mg) to a dry DCM solution (dichloromethane) at room temperature (10 ml), the air in the flask was replaced with nitrogen.
[0074] 2) An equivalent amount of 4-dimethylaminopyridine (DMAP) was added to the reaction solution and then stirred at room temperature for 1 hour under nitrogen protection. N,N'-dicyclohexylcarbodiimide (N,N'-dicyclohexylcarbodiimide) (100.8 mg) was added, and the reaction solution was reacted at room temperature for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the organic phase was extracted with dichloromethane solution to obtain a crude product. The reaction product was simply purified by silica gel column chromatography (dichloromethane:methanol=45:1) to obtain 133.1 mg of a red solid product.
[007...
Embodiment 3
[0081] Compound (I-3) is synthesized in the following manner
[0082]
[0083] (1) Synthesis of compound 3a:
[0084]Referring to the synthesis method of compound 1a, the starting material was changed to cy5 to finally obtain compound 3a.
[0085] Yield: 80.3%
[0086] 1 H NMR (600MHz, CDCl 3 )δ8.04(d, J=10.8Hz, 2H), 7.36(d, J=5.5Hz, 4H), 7.22(t, J=7.1Hz, 2H), 7.10(dd, J=15.2, 7.8Hz, 2H), 6.70(s, 1H), 6.20(t, J=12.9Hz, 2H), 5.50(d, J=4.4Hz, 1H), 4.59(d, J=7.7Hz, 1H), 4.30(s, 1H), 4.22(d, J=7.7Hz, 1H), 4.00(s, 2H), 3.93(d, J=5.8Hz, 1H), 3.64(d, J=14.6Hz, 4H), 3.28(d, J=9.3Hz, 1H), 2.25(s, 2H), 1.81(s, 2H), 1.72(d, J=12.3Hz, 14H), 1.51(d, J=15.1Hz, 2H), 1.49(s, 3H), 1.45(s,3H), 1.34(s,3H), 1.31(s,3H).
[0087] (2) Synthesis of Compound I-3:
[0088] Referring to the synthesis method of I-1, compound I-3 was finally obtained.
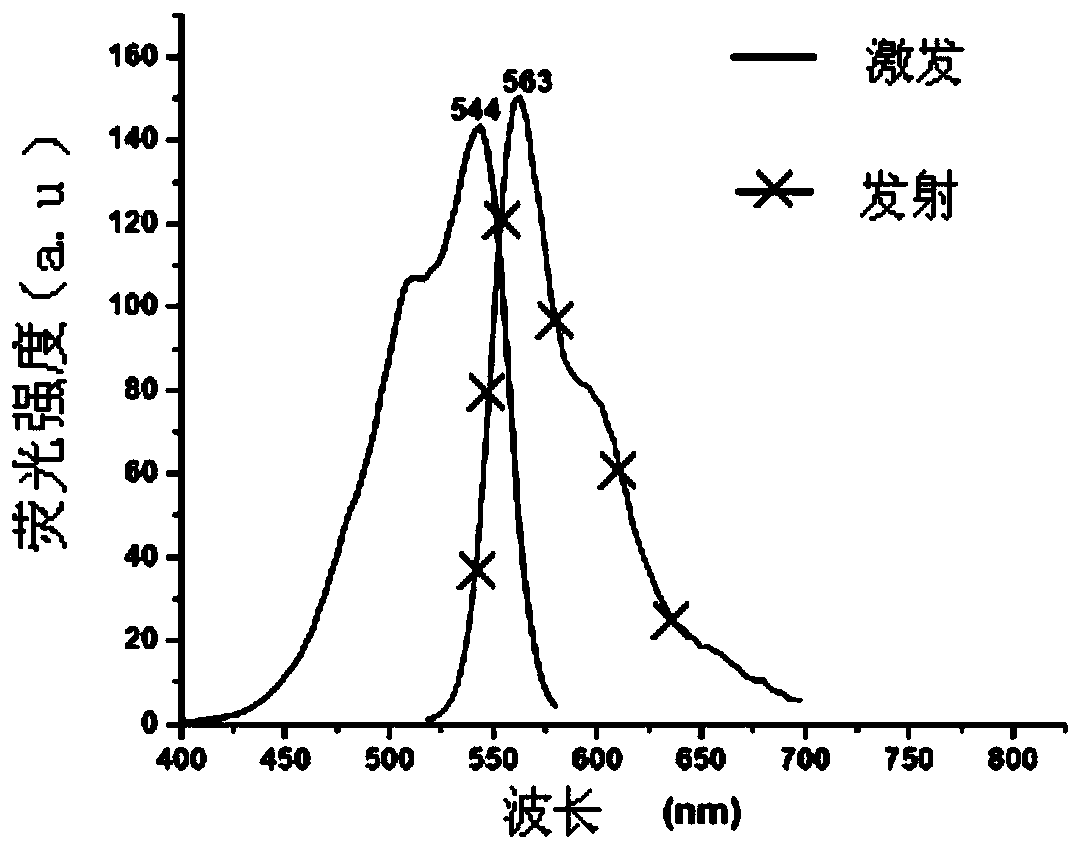
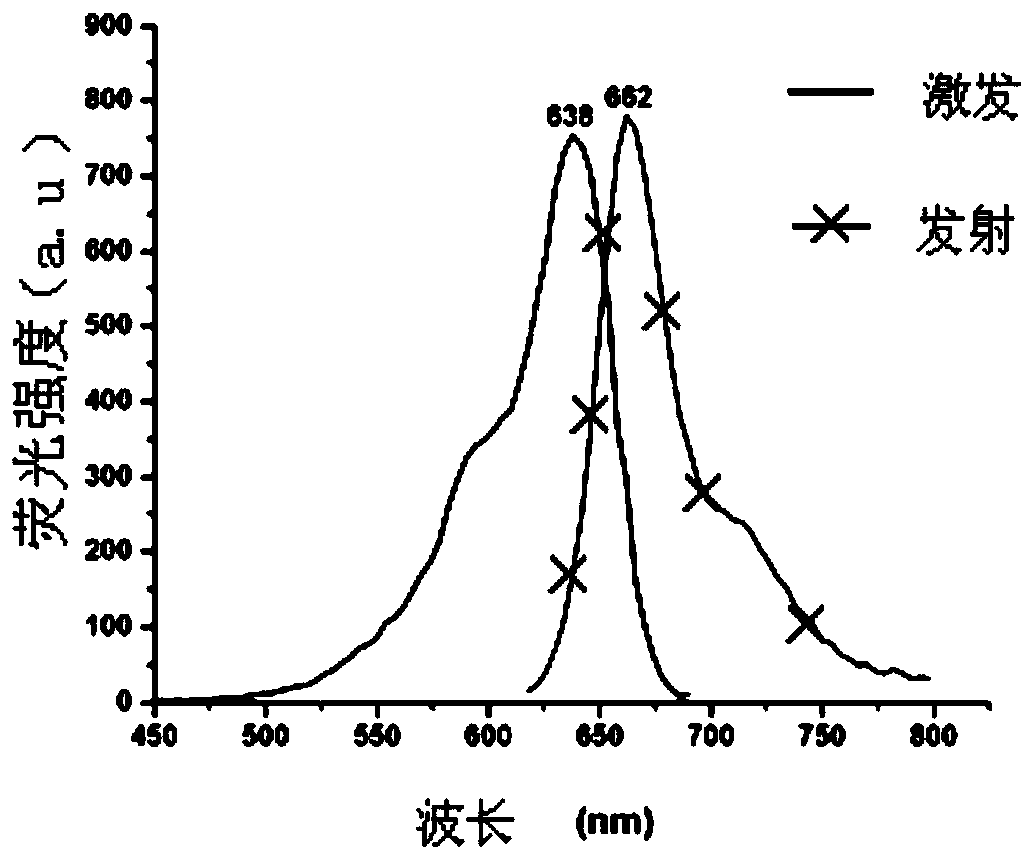
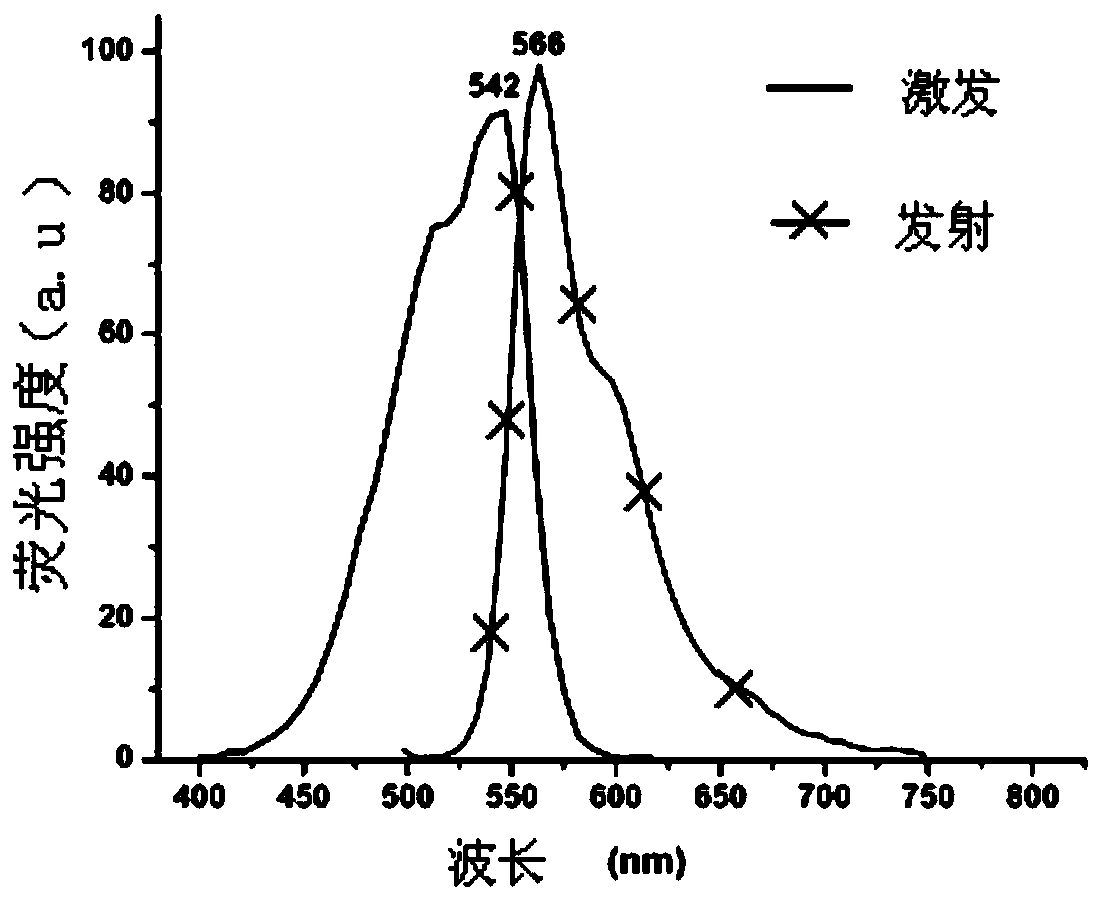
[0089] Yield: 68.1% (see Figure-2 for its excitation and emission spectra)
[0090] 1 H NMR (600MHz, MeOD) δ8.26 (t, J = 12.9Hz, 2H), 7.51 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com