Temozolomide slow-release system as well as preparation method and application thereof
A technology of temozolomide and sustained-release microspheres, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation and analysis of embodiment 1-temozolomide sustained-release microspheres:
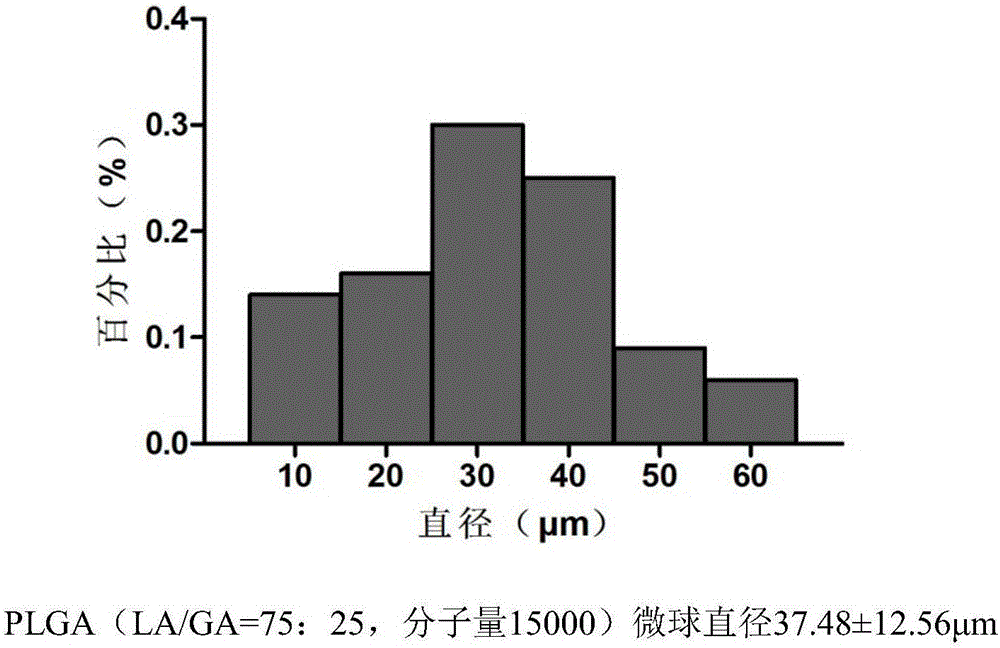
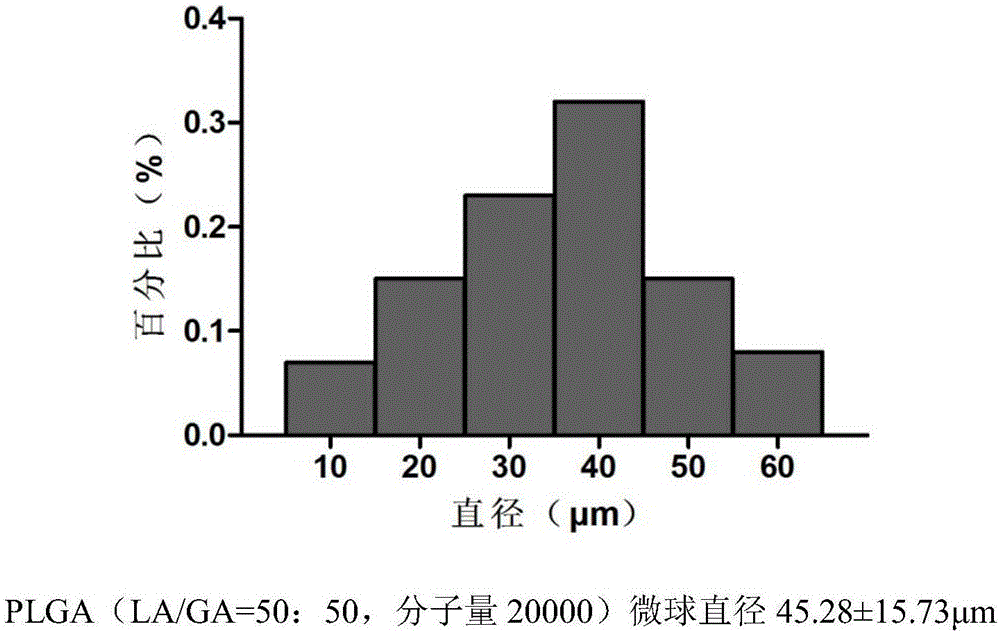
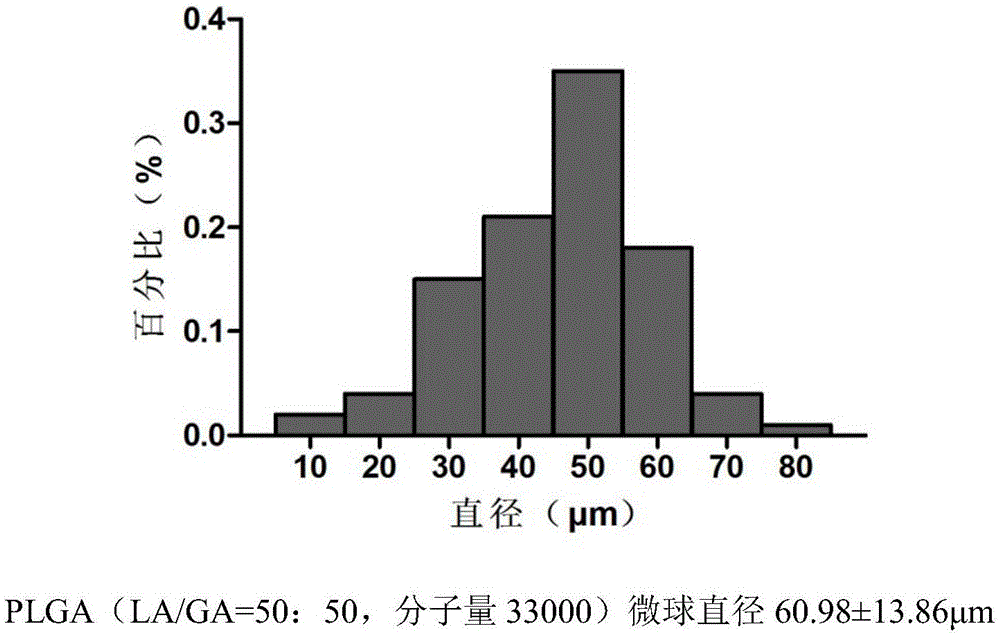
[0044] (1) Temozolomide-PLGA sustained-release microspheres were prepared by ultrasonic emulsification-solvent evaporation method: the molecular weights of PLGA were 15,000, 20,000, 33,000, and 36,000, respectively. TMZ crystal is dissolved in methanol, PLGA polymer is dissolved in dichloromethane, the two solutions are mixed, the obtained organic phase is stirred and emulsified in the solution containing polyvinyl alcohol, stirred at room temperature (800r / min), and the stirring speed becomes 350r after 30min / min until dichloromethane and methanol are completely volatilized. The obtained microspheres were washed with distilled water, screened to remove large particles, freeze-dried, and randomly packaged and refrigerated at 0°C. First observe the surface morphological characteristics of the four kinds of slow-release microspheres with a microscope, and then use a TOSHIBA800S scann...
Embodiment 2
[0055] Example 2-Study on the effect of TMZ-PLGA sustained-release microspheres on C6 glioma cells.
[0056] (1) Cell culture: Use a pipette gun to move glioma C6 cells into a culture flask, add 2.5ml of EMDM medium containing 10% calf serum, and put in CO 2 Inside the cell culture incubator. Cultures were changed every 2-3 days. Observe that the cell growth area accounts for more than 90% of the bottom of the culture flask, and then subculture. Remove the residual medium in the culture bottle, add 1ml of trypsin, observe with an inverted phase-contrast microscope, and find that the C6 cells become round and bright, immediately add 2ml of fresh medium containing 10% calf serum. Use a pipette to blow the cells off the bottom of the flask. Divide 1 bottle of cell suspension into 3 culture flasks equally, then add 2ml of 10% fresh medium of calf serum respectively, and finally put them into a constant temperature incubator for cultivation.
[0057] (2) Determination of cell...
Embodiment 3
[0064] Example 3-In vivo release of TMZ-PLGA sustained-release microspheres and their effects on rat C6 glioma.
[0065] (1) In vivo release study of TMZ-PLGA sustained-release microspheres: 7.5% TMZ-PLGA sustained-release microspheres were placed under the dura mater on the surface of the rat brain, respectively, at 1, 2, 3, 5, 8, 11, After 14 and 18 days, the tablets were taken out, and the peak areas corresponding to different TMZ drug concentrations were measured by high-pressure liquid chromatography, the drug concentration and the remaining TMZ content in the microspheres were calculated, and the TMZ release amount was calculated (see Figure 10-12 ). For the detection of TMZ drug concentration, the peak time is about 5min, which is relatively stable (such as Figure 10 ,11,12). Carry out linear regression with drug release rate (P%) to drug time (t) and draw regression equation to be P=4.2932t+30.5916, r2=0.9111, draw out the rat intracranial release curve of TMZ-PL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com