Preparation method of titanium bismuth oxyfluoride layered compound hierarchical-structure porous hollow spheres
A titanium oxyfluoride, hierarchical structure technology, applied in bismuth compounds, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., to achieve the effects of good reference, low production cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
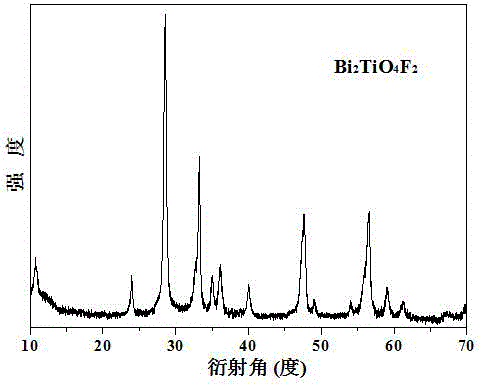
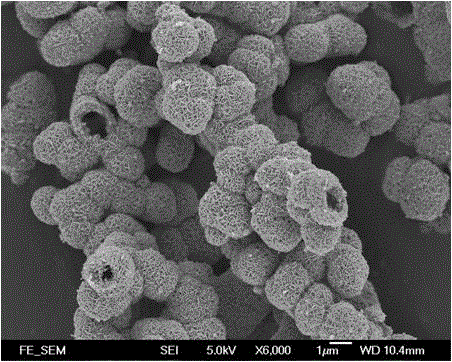
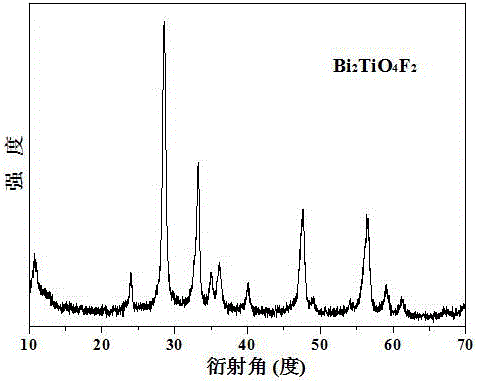
Image
Examples
Embodiment 1
[0026] 1) Weigh 1mmol of titanium dioxide, dissolve it in 5mL of hydrofluoric acid, remove the remaining hydrofluoric acid as much as possible by heating and evaporation, and finally dilute to 10mL with water.
[0027] 2) Weigh 2mmol of bismuth nitrate and dissolve it in 30mL of ethylene glycol.
[0028] 3) Add the solution in step 1) dropwise to the solution in step 2) under magnetic stirring, and mix well.
[0029] 4) The obtained mixed solution was transferred to a 50mL reaction autoclave, sealed, and reacted at a constant temperature of 150°C for 12 hours, and naturally cooled to room temperature after the reaction.
[0030] 5) The reaction product was suction filtered, washed alternately with water and ethanol several times, and dried at 60°C for 5 hours to obtain Bi 2 TiO 4 f 2 powder product.
Embodiment 2
[0032] 1) Weigh 1mmol of titanium dioxide, dissolve it in 5mL of hydrofluoric acid, remove the remaining hydrofluoric acid as much as possible by heating and evaporation, and finally dilute to 13mL with water.
[0033] 2) Weigh 2mmol of bismuth nitrate and dissolve it in 26mL of ethylene glycol.
[0034] 3) Add the solution in step 1) dropwise to the solution in step 2) under magnetic stirring, and mix well.
[0035] 4) The obtained mixed solution was transferred to a 50mL reaction autoclave, sealed, and reacted at a constant temperature of 150°C for 12 hours, and naturally cooled to room temperature after the reaction.
[0036] 5) The reaction product was suction filtered, washed alternately with water and ethanol several times, and dried at 60°C for 5 hours to obtain Bi 2 TiO 4 f 2 powder product.
Embodiment 3
[0038] 1) Weigh 1mmol of titanium dioxide, dissolve it in 5mL of hydrofluoric acid, remove the remaining hydrofluoric acid as much as possible by heating and evaporation, and finally dilute to 15mL with water.
[0039] 2) Weigh 2mmol of bismuth nitrate and dissolve it in 25mL of glycerol.
[0040] 3) Add the solution in step 1) dropwise to the solution in step 2) under magnetic stirring, and mix well.
[0041] 4) The obtained mixed solution was transferred to a 50mL reaction autoclave, sealed, and reacted at a constant temperature of 180°C for 12 hours, and naturally cooled to room temperature after the reaction.
[0042] 5) The reaction product was suction filtered, washed alternately with water and ethanol several times, and dried at 60°C for 5 hours to obtain Bi 2 TiO 4 f 2 powder product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com