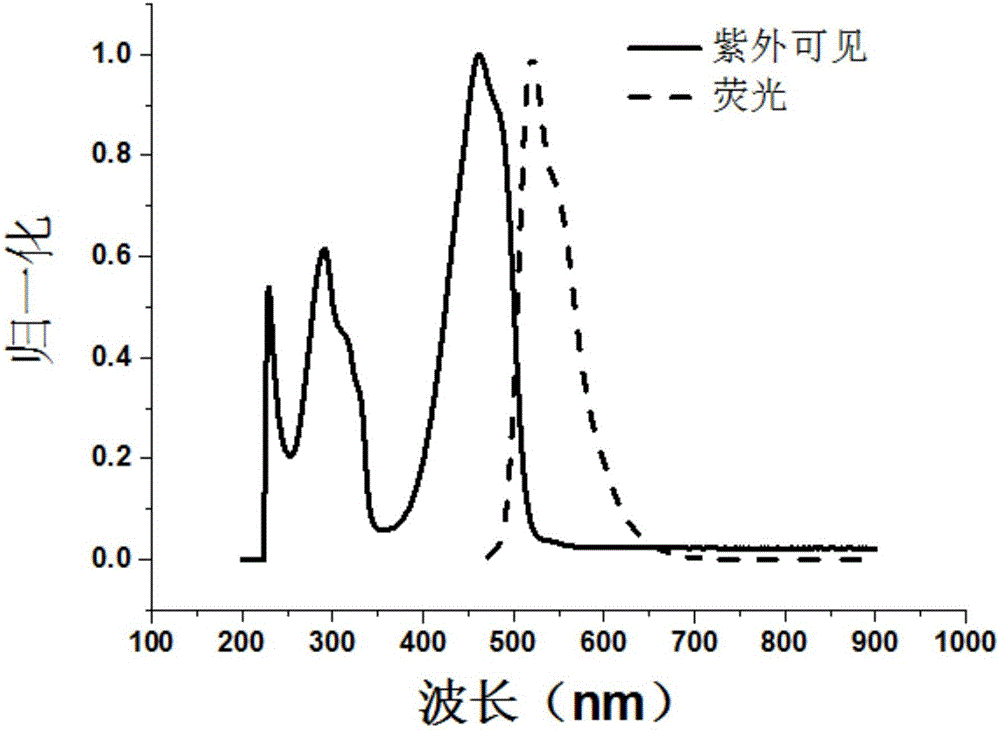
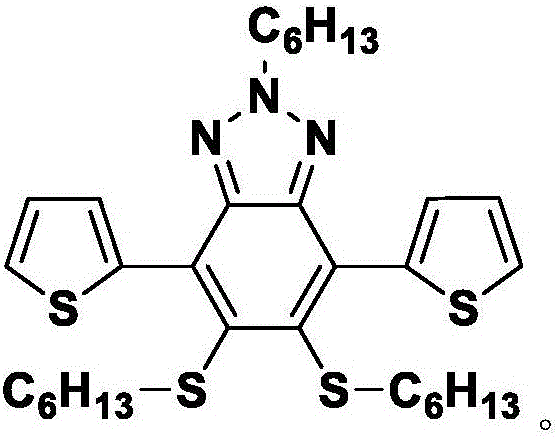
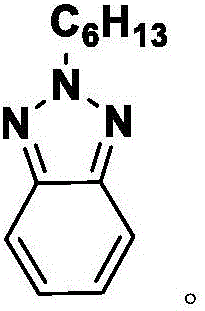
Benzotriazole compound synthesis method
A technology of benzotriazoles and synthesis methods, which is applied in the direction of organic chemistry, can solve the problems of not involving the structure of benzene rings, etc., and achieve the effect of changing optical and electrical properties, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of 2-hexyl-benzo[d][1,2,3]triazole
[0022]
[0023] Add benzotriazole (10.00g, 83.94mmol), sodium tert-butoxide (8.47g, 105.77mmol) and n-bromohexane (16.21g, 98.21mmol) successively in a two-necked flask with a molar ratio of 1:1.17:1.26 , then add 100mL of methanol solution, the solution is heated to 80 ° C for 12 hours; stop the reaction, distill off the solvent methanol under reduced pressure to obtain the sample, and then dissolve the sample in dichloromethane, respectively, with deionized water and saturated saline Wash the dichloromethane solution, add anhydrous magnesium sulfate to dry the dichloromethane solution, filter and concentrate the dichloromethane solution, and the concentrated product is separated by silica gel chromatography (eluent is petroleum ether / dichloromethane, v / v= 2:1), and dried in vacuo to obtain 2-hexyl-benzo[d][1,2,3]triazole (7.20 g, 35.42 mmol) as a colorless liquid with a yield of 42%.
Embodiment 2
[0024] Example 2: Preparation of 2-hexyl-4,7-dibromo-benzo[d][1,2,3]triazole
[0025]
[0026] Dissolve 2-hexyl-benzo[d][1,2,3]triazole (3.45g, 16.97mmol) in 35mL of hydrobromic acid, raise the temperature to 100°C and stir at constant temperature for 1 hour, slowly drop into the molar ratio It is liquid bromine (15.46g, 96.72mmol) of 1:2.7, after dripping, it is heated to 135°C and refluxed at this temperature for 12 hours; to stop the reaction, add saturated aqueous sodium thiosulfate solution, extract with ethyl acetate, and use Wash the ethyl acetate solution with deionized water and saturated brine, add anhydrous magnesium sulfate to dry the ethyl acetate solution, filter and concentrate the ethyl acetate solution, and the concentrated product is separated by silica gel chromatography (eluent is petroleum ether / distillate Chloromethane, v / v=2:1), dried in vacuo to give light yellow liquid 2-hexyl-4,7-dibromo-benzo[d][1,2,3]triazole (4.90g, 13.57mmol) , and its yield w...
Embodiment 3
[0027] Example 3: Preparation of 2-hexyl-4,7-dibromo-5,6-dinitro-benzo[d][1,2,3]triazole
[0028]
[0029] Slowly drop 2-hexyl-4,7-dibromo-benzo[d][1,2,3]triazole (7.30g, 20.22mmol) into 60mL of mixed fuming nitric acid and concentrated sulfuric acid (fuming Nitric acid / concentrated sulfuric acid, v / v=1:1), keep the temperature of the reaction solution within 0°C, and stir for 3 hours; stop the reaction, pour the solution into ice water slowly, extract with ethyl acetate, and use deionized water and Wash the ethyl acetate solution with saturated brine, add anhydrous magnesium sulfate to dry the ethyl acetate solution, filter and concentrate the ethyl acetate solution, and the concentrated product is separated by silica gel chromatography (eluent is petroleum ether / dichloromethane, v / v=2:1), dried under vacuum to obtain light yellow solid 2-hexyl-4,7-dibromo-5,6-dinitro-benzo[d][1,2,3]triazole (5.7g , 12.64mmol), the yield was 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com