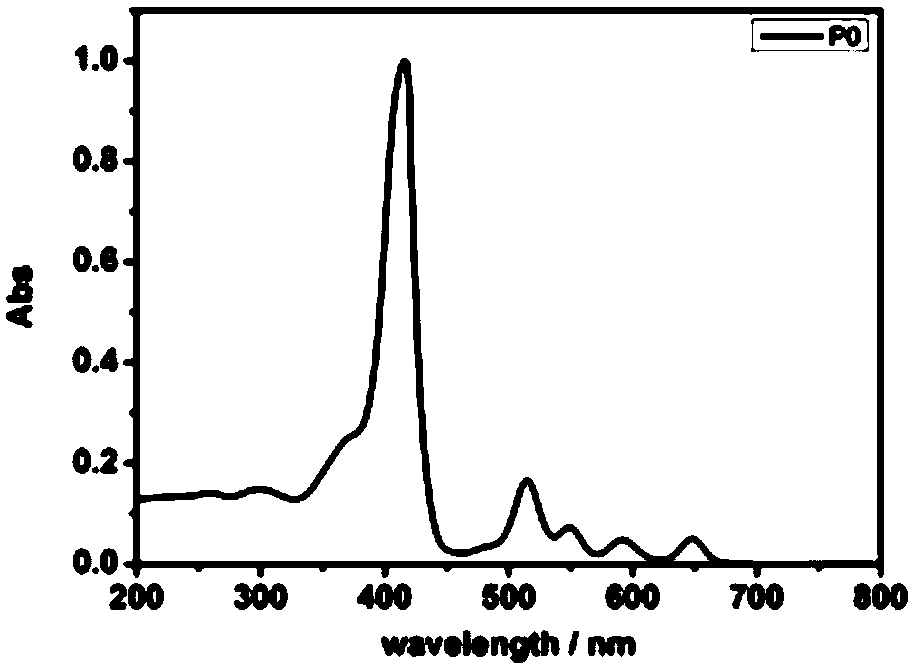
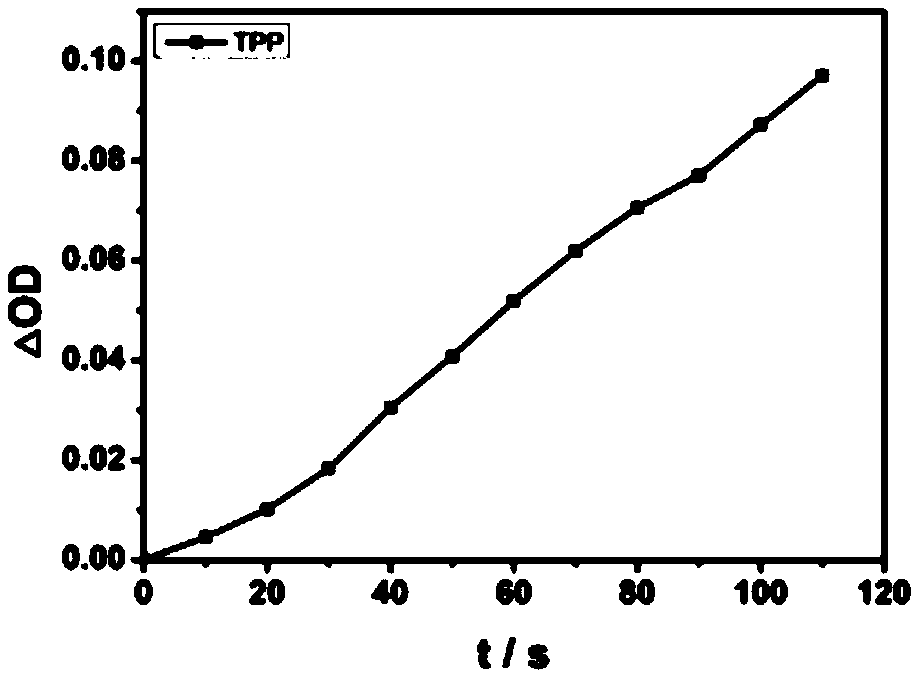
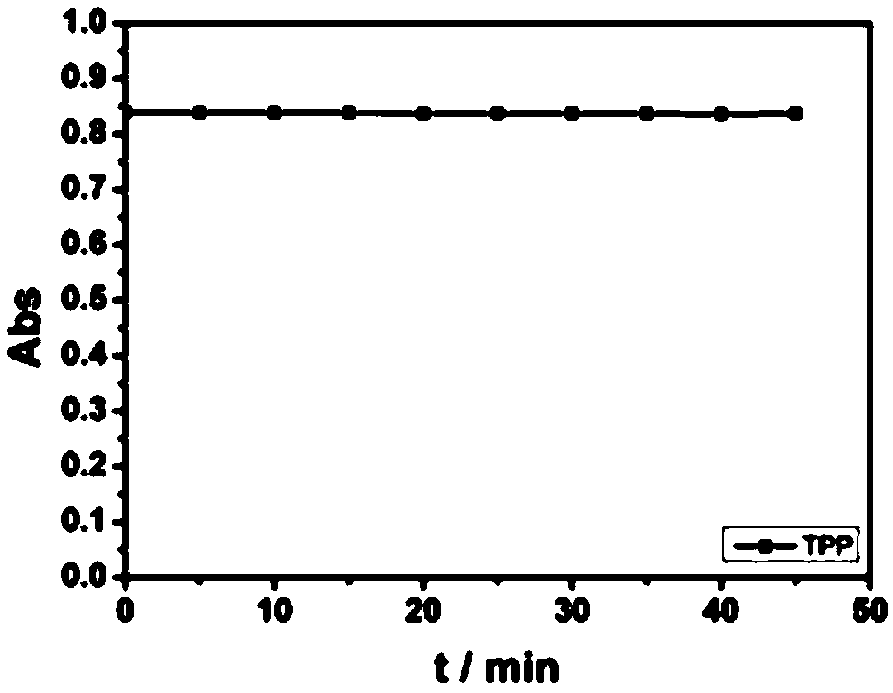
Porphyrin compound with good water solubility and near-infrared absorption and its preparation method and application
A porphyrin compound, water-soluble technology, applied in the field of water-soluble, near-infrared-absorbing porphyrin compound and its preparation, can solve unsolved problems such as porphyrin solubility, achieve high singlet oxygen yield, low dark toxicity, phototoxic short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] with R 1 structured as R 2 The structure is R 2 = R 1 or choose n 1 =3, M=Zn as an example. Its specific synthetic route is as follows:
[0065] a, the preparation of aldehyde
[0066]
[0067] Take triethylene glycol monomethyl ether (4.4mL, 27.6mmol) and p-toluenesulfonyl chloride (5.7g, 30mmol) and dissolve in 80mL of dichloromethane, and place in a 250mL round bottom flask, then add triethylamine (11.1 mL, 82.5mmol), the mixed solution was stirred and reacted at room temperature for 12h. After the reaction was completed, the solvent was removed by rotary evaporation to obtain an oily mixture. Using ethyl acetate:petroleum ether=2:1 (V / V) as eluent, the product was purified by column chromatography to obtain 9 g of a colorless oily liquid (88 %). 1 HNMR (CDCl 3 ,500MHz): δ=7.81(d,J=10.0Hz,2H),7.34(d,J=10.0Hz,2H),4.17(t,J=5.0Hz,2H),3.69(t,J=5.0Hz ,2H),3.63-3.60(m,6H),3,54(t,J=4.0Hz,2H),3.38(s,3H),2.45(s,3H).MALDI-TOF MS:m / z calcd for C 14 h 22 o 6...
Embodiment 2
[0088] with R 1 structured as R 2 The structure is R 2 = R 1 or choose n 2 =3; M=Zn as an example. Its specific synthetic route is as follows:
[0089] a, the preparation of aldehyde
[0090]
[0091] Weigh 3,4-dihydroxybenzaldehyde (2.76g, 20mmol) and tosylate triethylene glycol monomethyl ether (8.06g, 48mmol) and dissolve in 120mL DMF solution, and place in a 500mL round-bottomed flask, and then Join K 2 CO 3 (8.28g, 60mmol) and LiBr (1.74g, 20mmol), the mixture was heated to 100°C in an oil bath and stirred for 48h. 2M H was added after the reaction 2 SO 4 (200mL) to quench the reaction, after 30min to finish the reaction cooling, extract the organic layer with dichloromethane and deionized water; the organic layer was washed with anhydrous MgSO 4 Dry, filter and remove the solvent by rotary evaporation. Using dichloromethane:methanol=99:1 (V / V) as eluent, the product was purified by column chromatography to obtain 6.6 g (62%) of light yellow oily prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com