Novel FSH-Fc fusion protein as well as preparation method and application thereof
One kind of fusion protein, another technology, application in assisted reproduction, gonadotropin, new long-acting gonadotropin and its preparation field, can solve problems such as limited application of clinical treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
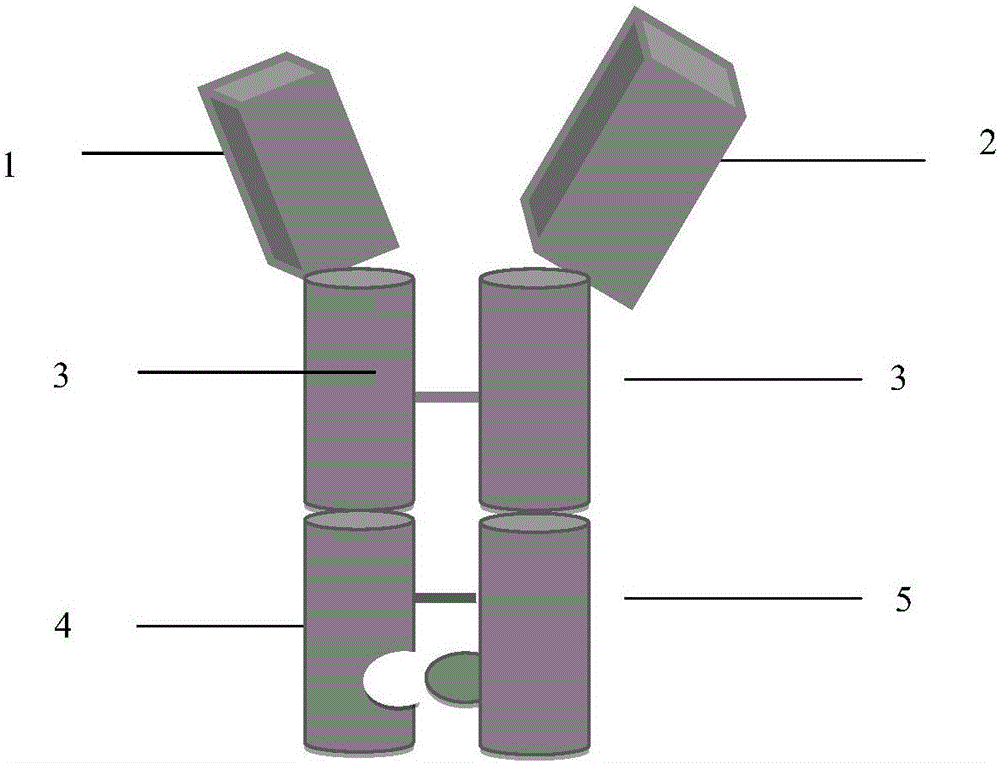
[0033] Example 1. Construction of FSHα-Fch-FSHβ-Fck heterodimer expression plasmid
[0034] According to the amino acid sequence of the FSHα subunit (Gene bank No.NP_000726.1), the amino acid sequence of the human FSHβ subunit (Gene bank No.NP_000501.1) in the NCBI database, and the locked Fc shown in SEQ ID NO:1 Chain amino acid sequence (containing T148S, L150A, Y189V mutations, forming a small side chain structure) and the amino acid sequence of the button Fc chain shown in SEQ ID NO: 2 (containing T148W mutation, forming a large side chain structure), respectively artificial The coding DNA sequences of FSHα-Fch (SEQ ID NO:3) and FSHβ-Fck (SEQ ID ID NO:4) were synthesized. When synthesizing, restriction endonuclease SapI restriction endonuclease SapI restriction endonuclease restriction sites were introduced in the upstream and downstream of the coding sequence respectively.
[0035] After the coding DNA is synthesized, use the SapI restriction site to insert FSHα-Fch into...
Embodiment 2
[0037] Example 2. Expression and purification of FSHα-Fch-FSHβ-Fck heterodimeric protein
[0038] 2.1 Stable expression of FSHα-Fch-FSHβ-Fck heterodimer
[0039] CHO-K1 (ATCC, CCL-61) cells acclimatized to serum-free medium were cultured in 5×10 6 The density of cells / ml was inoculated in 30ml CD-CHO medium for culture. After 24 hours, the cell density should reach 1~2×10 6 cells / ml. Centrifuge the cell suspension at 800rpm for 5min, discard the supernatant, wash the cells twice with 50ml CD CHO medium, discard the supernatant, and resuspend in 1ml CD CHO medium. Cells and plasmids were divided into 1 × 10 7 Cells: 40 μg DNA was mixed in proportion, and electroporation transfection parameters were set: voltage 300V; capacitance 900 μF, and electric pulse was performed. Plate well 100 μL. After 24 hours, 100 μL of MSX working solution was added for pressurized screening. After the cell plate was cultured for 3 weeks, cell clones formed in the wells of the plate. Select m...
Embodiment 3
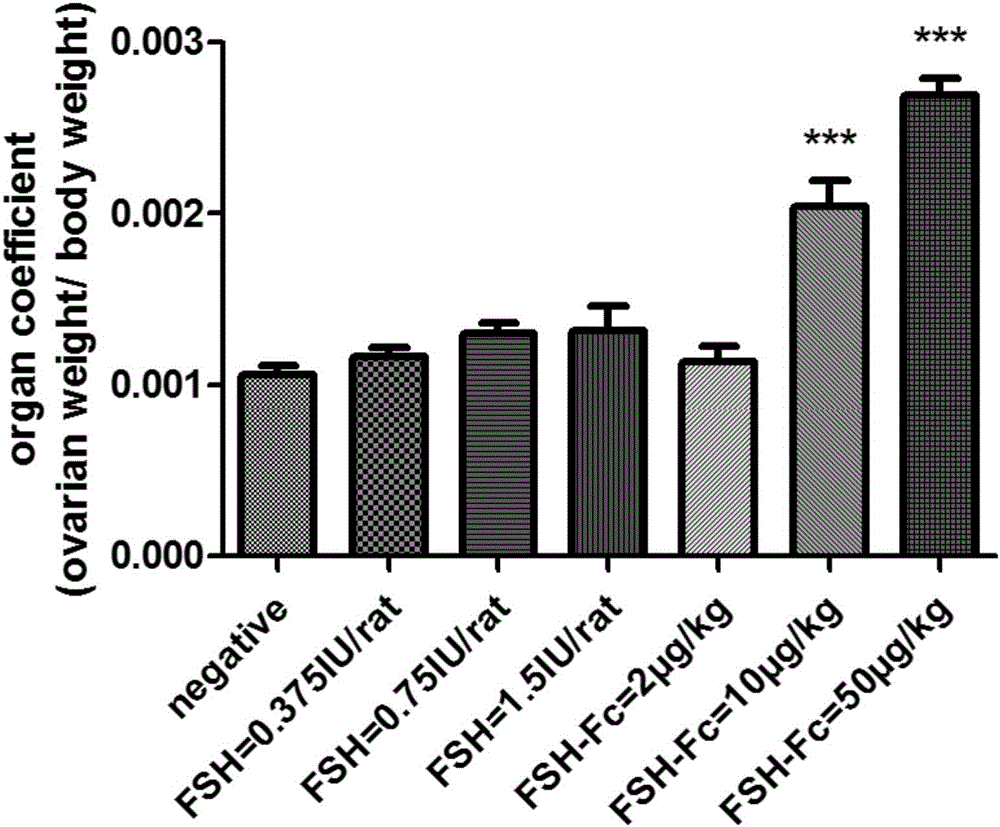
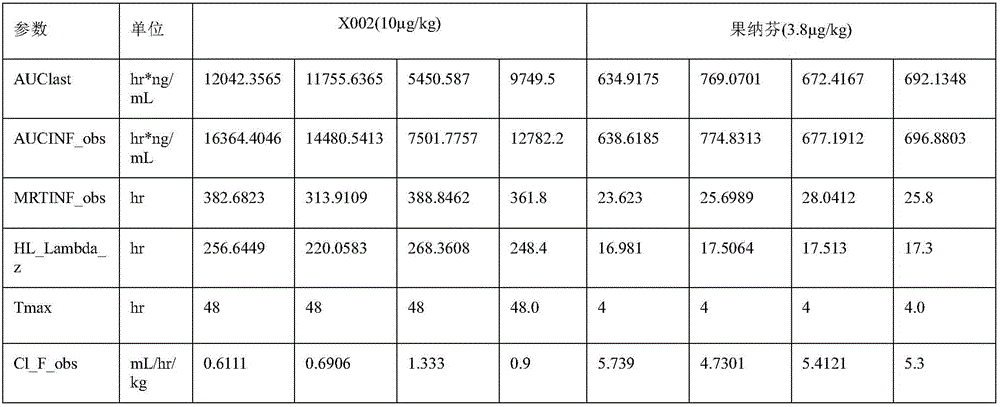
[0042] Example 3 Detection of Biological Activity of FSHα-Fch-FSHβ-Fck Heterodimer
[0043] According to Chinese Pharmacopoeia, the biological activity of FSH recombinant protein was determined by rat ovary weight gain method. HCG is used to assist the test FSH drug to stimulate immature 21-day-old female rats to trigger ovarian follicle growth and oocyte production. By measuring the weight of the ovary at the end of administration, the biological activity of the test drug can be clearly evaluated. Get 3-week-old SPF female rats with a body weight of 40-60g, divide them into 7 experimental groups according to their average body weight, set up a blank control group, high, medium and low dose positive control drug dosage groups and high, medium and low dose test drug group. The control drug Lishenbao (purchased from Livzon Pharmaceutical) was administered once a day, and the test sample FSHα-Fch-FSHβ-Fck was administered only once, and the administration method was subcutaneous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com