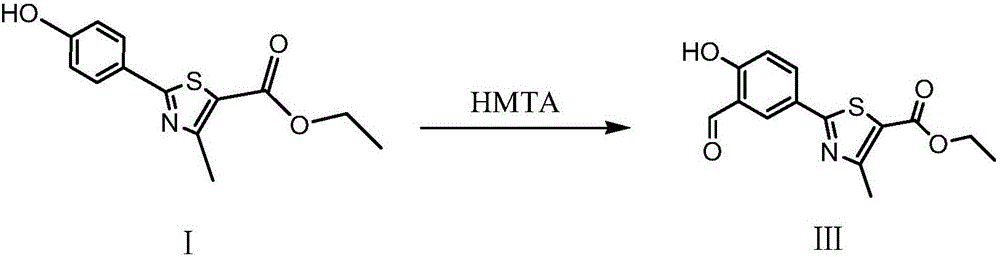
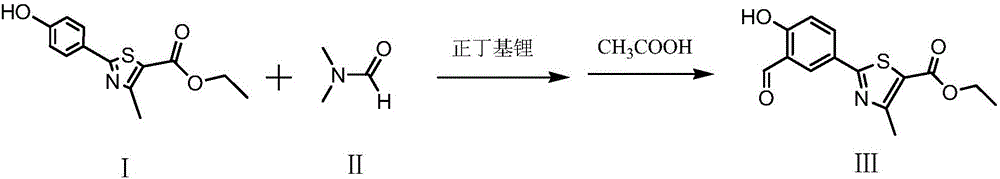
Synthesis method of ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
A technology of hydroxyphenyl and methylthiazole, which is applied in the field of drug synthesis, can solve problems such as unfavorable industrial production, inconvenient production operation, and personnel allergies, and achieve the effects of simple production methods, improved work efficiency, and reduced allergenic factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Add 600ml of tetrahydrofuran and 70g of ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate into a 2000ml reaction flask, then pass through nitrogen protection, and when the temperature drops to -10°C, slowly drop Add 536ml of 1mol / L n-butyllithium n-hexane solution, after the dropwise addition is complete, stir at -10°C for 15 minutes, then add 22.3g of N,N-dimethylformamide, and stir for 30 minutes;
[0022] (2) Add 48g of glacial acetic acid under temperature control below 10°C, stir for 10 minutes, control the temperature of the water bath at 40°C to concentrate the reaction solution under reduced pressure to dryness, then add 1000ml of purified water to it, add 90g of sodium bicarbonate, stir for 30 minutes, and add to the reaction solution Add 1200ml of ethyl acetate to the solution and extract twice, combine the organic layers, control the temperature of the water bath at 50°C and concentrate the organic solvent under reduced pressure until no fraction fl...
Embodiment 2
[0024] (1) Add 600ml of tetrahydrofuran and 70g of ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate into a 2000ml reaction flask, then pass through nitrogen protection, and when the temperature drops to -10°C, slowly drop Add 530ml of n-butyllithium n-hexane solution of 1mol / L, after the dropwise addition is complete, stir at -10°C for 20 minutes, then add 22.0g of N,N-dimethylformamide, and stir for 35 minutes;
[0025] (2) Add 45g of glacial acetic acid at 0-10°C of temperature control, stir for 15 minutes, control the water bath temperature at 40°C to concentrate the reaction solution under reduced pressure to dryness, then add 1000ml of purified water thereto, add 85g of sodium bicarbonate, stir for 30 minutes, and Add 1200ml of ethyl acetate to the reaction solution and extract twice, combine the organic layers, control the temperature of the water bath at 50°C to concentrate the organic solvent under reduced pressure until no fraction flows out, add 210ml of isopr...
Embodiment 3
[0027] (1) Add 600ml of tetrahydrofuran and 70g of ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate into a 2000ml reaction flask, then pass through nitrogen protection, and when the temperature drops to -10°C, slowly drop Add 550ml of n-butyllithium n-hexane solution of 1mol / L, after the dropwise addition is complete, stir at -10°C for 12 minutes, then add 23.0g of N,N-dimethylformamide, and stir for 30 minutes;
[0028] (2) Add 50 g of glacial acetic acid under temperature control below 10°C, stir for 10 minutes, control the water bath temperature at 40°C to concentrate the reaction solution under reduced pressure to dryness, then add 1000ml of purified water to it, add 95g of sodium bicarbonate, stir for 30 minutes, and add to the reaction solution Add 1200ml of ethyl acetate to the solution and extract twice, combine the organic layers, control the temperature of the water bath at 50°C and concentrate the organic solvent under reduced pressure until no fraction flows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com