Preparation method and application of mesenchymal stem cell cytokines
A technology of mesenchymal stem cells and cytokines, which is applied in the field of preparation of mesenchymal stem cell cytokines, can solve the problems that cytokine compositions cannot simulate cytokine types and proportions, adverse reactions, etc., and achieve simple preparation methods and inhibition of degradation , reduce the effect of rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 In vitro expansion and identification of mesenchymal stem cells
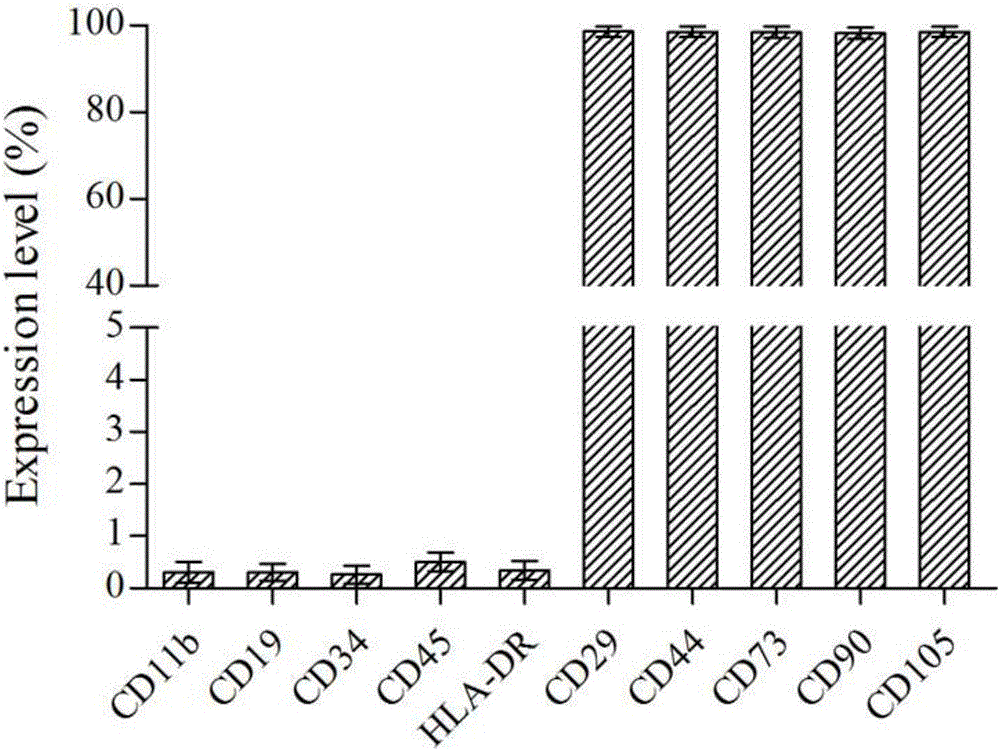
[0028] After digesting the P5 generation placental mesenchymal stem cells with a confluence of 90% (tissue attachment method or enzyme digestion method), counting, and preparing a density of 1 × 10 6 cells / mL cell suspension, then filtered with a 100 μm cell mesh, and then 1 mL of the filtrate was taken, and 10 μL of fluorescently labeled cell surface marker antibodies were added to the filtrate, and incubated at 4 °C for 20 min in the dark. .
[0029] After incubation, wash once with 0.9% normal saline (4°C), centrifuge at 1200r / min for 5min, discard the supernatant, add 250μL 0.9% normal saline to the precipitate and mix well, then add 250μL 1% paraformaldehyde Fix, then adopt the flow cytometer of Beckman Company to carry out detection and analysis, the detection result sees figure 1 .
[0030] The antibodies used are CD11b-FITC, CD19-ECD, CD29-FITC, CD34-PE, CD44-FITC, CD45-PC7, CD73-PE, ...
Embodiment 2
[0032] Example 2 Preparation of clinical grade mesenchymal stem cell cytokines
[0033] Inoculate the mesenchymal stem cells into the culture vessel, add the medium, and then place it at 37°C, 5% CO 2 subculture under the following conditions; medium components: FBS with a volume concentration of 20% and GlutaMAX with a volume concentration of 2% TM -I, the rest is DMEM; after the confluence of P5 mesenchymal stem cells reaches 80-90%, adjust the oxygen concentration in the culture container to 1% (v / v), and then continue to culture the cells overnight;
[0034] Digest the overnight cells with 0.25% trypsin-EDTA solution for 2-3min, then centrifuge at 20°C and 2000r / min for 3min, remove the supernatant, wash the precipitate with 0.9% normal saline for 2-3 times and collect cells, and the cell viability was detected, and the cells were counted.
[0035] Adjust the cell concentration to 1 x 10 with 0.9% saline 8 cells / mL, then add 3 mg / mL EDTA and 10 mg / mL Vc, mix well, then ...
Embodiment 3
[0039] Example 3 Preservation Validity Experiment of Mesenchymal Stem Cell Cytokines
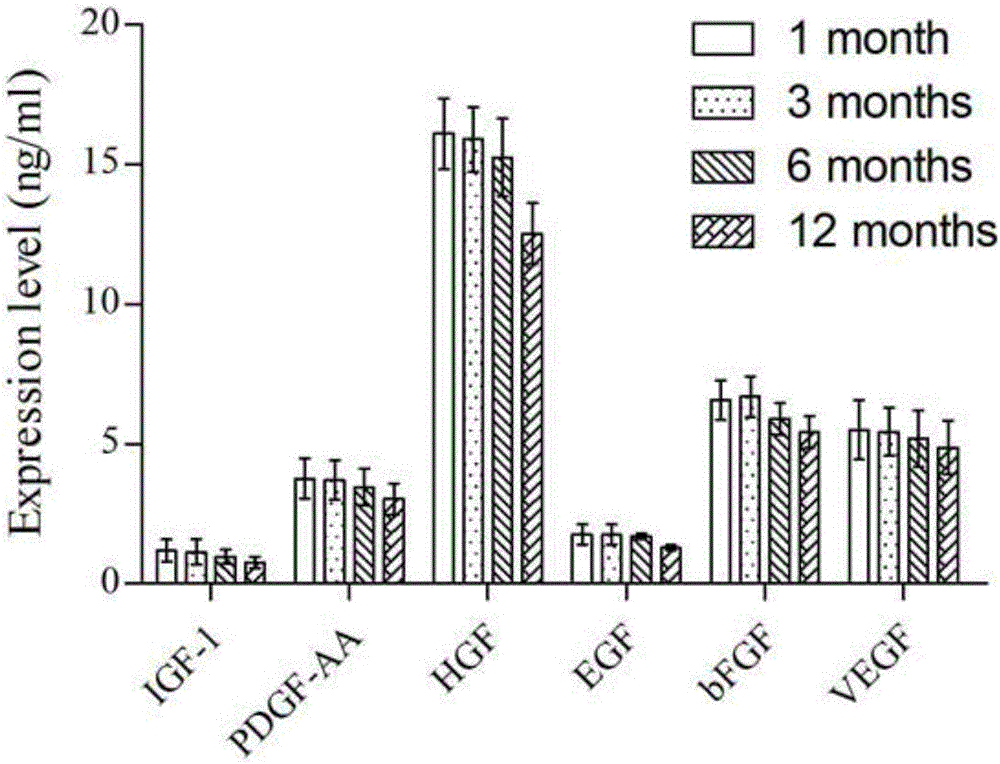
[0040] The prepared mesenchymal stem cell cytokines were stored at -80°C for 1 month, 3 months, 6 months, and 12 months. Freshly prepared mesenchymal stem cell cytokines were used as the control group, and ELISA kits were used to According to the instruction manual, various cytokines (EGF, FGF, IGF-1, HGF, PDGF-AA, VEGF) were detected, and the kits were purchased from R&D company. The experimental results are shown in image 3 .
[0041] Depend on image 3 It can be seen that, taking the cytokine content frozen for 3 months as a reference, the decrease of cytokines is not obvious within 6 months of frozen storage (P>0.05). When the frozen storage time increases to 12 months, the cytokine content There is a small amount of loss (P<0.01), so the validity period of the stem cell factor preparation is recommended to be 12 months.
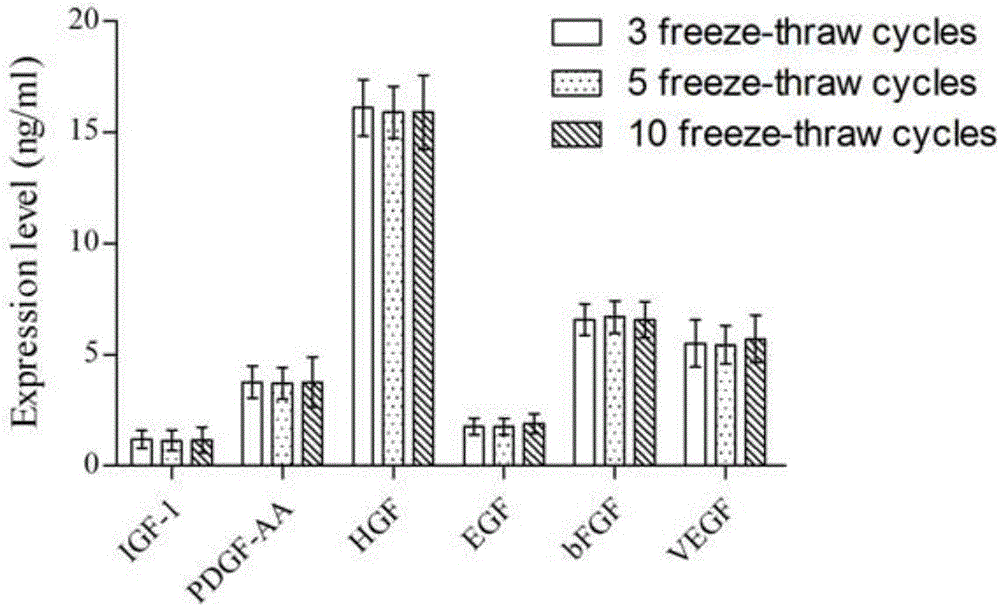
[0042] The mesenchymal stem cell cytokine prepared by the inv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com