Method for preparing ginsenoside-Rd through immobilization of cellulase and enzymolysis of ginsenoside-Rb1 by virtue of covalent cross-linking process
A technology of ginsenoside and cellulase, applied in the direction of fermentation, can solve the problems of weak enzyme binding force, easy inactivation contact surface area, etc., and achieve the effects of high selectivity, convenient continuous and automatic operation, and saving production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Covalent immobilization process of cellulase.
[0023] Weigh 0.25g of carrageenan (purchased from Beijing Suolaibao Company) and add it to 25ml of distilled water at a temperature of 65°C. Stir with a magnetic stirrer at a speed of 300rpm for 1h to obtain a carrageenan suspension; take 4°C 50ml of 2% KCl solution was placed in a beaker, stirred on a magnetic stirrer at a speed of 300rpm, and the carrageenan suspension was sucked up with a 5ml syringe, and slowly dropped into it (the drop distance was fixed at 6 cm during the dropping process, and the dropping distance was 6 cm. The speed is 80 drops / min), and transparent hemispherical gel beads are formed instantaneously, and placed in 2% KCl solution for 3 hours to make gel beads with a concentration of 1% carrageenan; take 1g of carrageenan gel beads, add 10ml concentration For 1% polyethyleneimine solution, add 10% NaOH solution dropwise to adjust the pH value to 10, put it on a roller mixer for mixing and amination ...
Embodiment 2
[0025] Covalent immobilization process of cellulase.
[0026] Weigh 0.5g of carrageenan and add it to 25ml of distilled water. Stir with a magnetic stirrer at a temperature of 65°C at a speed of 300rpm to obtain a suspension of carrageenan; take 50ml of 2% KCl solution at 4°C and place it in a beaker , stirred on a magnetic stirrer with a rotation speed of 300rpm, and sucked the carrageenan suspension with a 5ml syringe, and slowly dropped it into it (during the dropping process, the fixed drop distance was 6 cm, and the dropping speed was 80 drops / min), and the Transparent hemispherical gel beads, and placed in 2% KCl solution for 3 hours to make 1% carrageenan gel beads; take 1 g of carrageenan gel beads, add 10 ml of 0.5% polyethyleneimine solution , add 10% NaOH solution dropwise to adjust the pH value to 8, place it on a roller mixer for amination for 3 hours, wash with distilled water 3 times, and clean the residual polyethyleneimine; then add it to 10 ml of 3% glutarald...
Embodiment 3
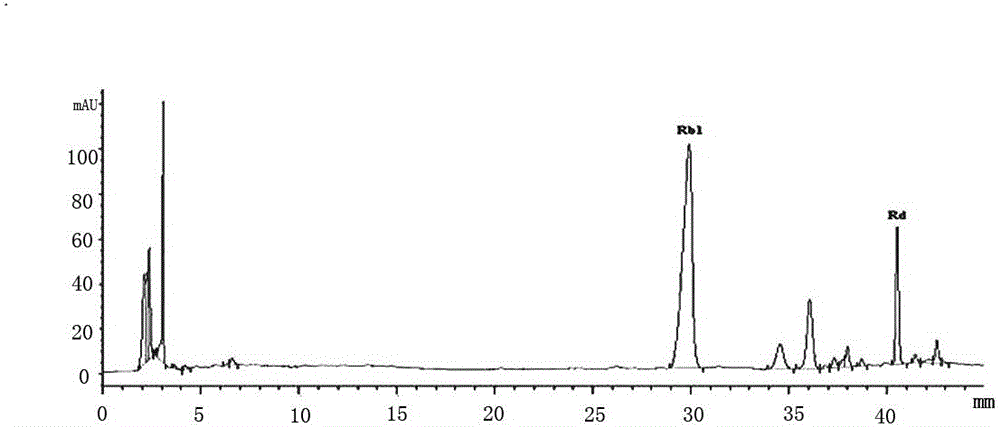
[0031] Conversion of Ginsenoside-Rb from Panaxadiol Group Saponins by Immobilized Enzyme 1 .
[0032] 1. Preparation of saponin samples of American ginsengdiol group.
[0033] American ginseng dry root and rhizome coarse powder are decocted and extracted with water, the residue is filtered off, the water is concentrated under reduced pressure to the concentration of American ginseng, and then separated on D 101 macroporous adsorption resin. The amount of macroporous adsorption resin is 1.5 times the concentrated solution. Adsorb at 55°C for 12 h, wash with water until there is no sweetness, then desorb with 30% and 65% ethanol solutions in sequence, collect 65% of the eluent, spin the solvent under reduced pressure, and further dry under reduced pressure to obtain American ginseng diol Saponin dry powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
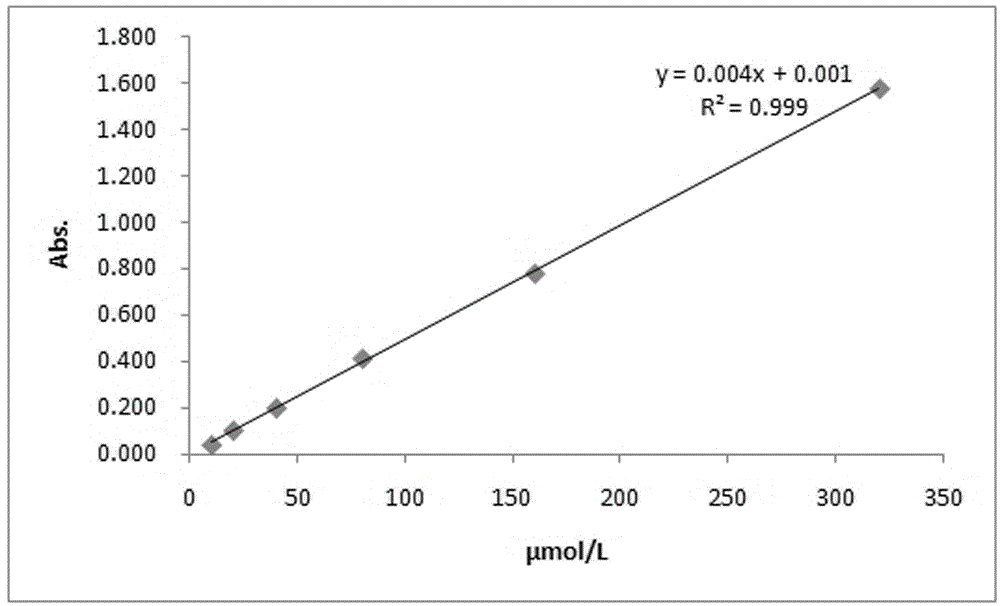
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com