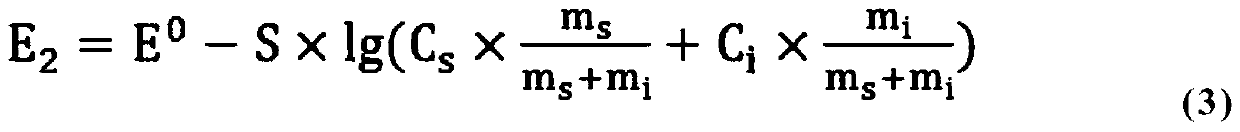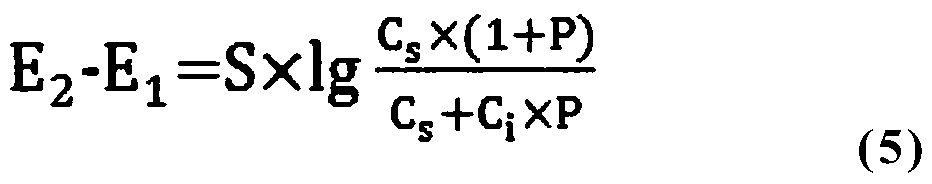A method for detecting fluoride ion content in perfluoroalkylsulfonylimide salts
A technology of perfluoroalkylsulfonylimide salts and fluoride ions, which is applied in the field of analytical chemistry and can solve problems such as the inability to measure fluoride ion content and the interference of fluoride ion electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Prepare total ionic strength adjustment buffer solution TISAB solution: prepare potassium nitrate-trisodium citrate mixed solution, wherein potassium nitrate 1mol / L, trisodium citrate 1mol / L, and adjust pH with glacial acetic acid to be 6~7;
[0057] (2) Preparation of fluoride ion standard stock solution (concentration: 1000mg / kg): Accurately weigh 0.2210g of the reference reagent NaF (dried at 105-110°C for 2 hours, and cooled to room temperature in a drying oven), and dilute it with deionized water to 100g;
[0058] (3) Preparation of fluoride ion standard test solution:
[0059] Preparation of fluoride ion standard test solution 1 (concentration is 1mg / kg): Accurately weigh 0.0500g of fluoride ion standard stock solution, add 10mL total ionic strength adjustment buffer solution, then add 3.5g potassium nitrate, and finally dilute to 50g, dissolve and mix well;
[0060] Preparation of fluoride ion standard test solution 2 (concentration is 100mg / kg): Accurately w...
Embodiment 2
[0067] (1) prepare total ionic strength adjustment buffer solution TISAB solution: with example 1;
[0068] (2) preparation of fluoride ion standard stock solution (concentration is 1000mg / kg): same as example 1;
[0069] (3) preparation of fluoride ion standard test solution: with example 1;
[0070] (4) Sample analysis
[0071] Fluoride ion standard solution 1 and fluoride ion standard solution 2 were tested with a fluoride ion meter, and the electrode constant S was obtained as 58.57 according to their respective concentrations and potential values;
[0072] Weigh 12.2993gNa + [N(CF 3 SO 2 )(C 2 f 5 SO 2 )] - Sodium perfluoroalkylsulfonylimide was added to a beaker, and 10mL of total ionic strength adjustment buffer solution was added, then diluted to 50.3565g with distilled water, and mixed evenly to obtain a sample solution. The mass of the solution was m s , the solution potential E was measured with a fluoride ion meter 1 is 100.2mV;
[0073] Then add fluorid...
Embodiment 3
[0076] (1) prepare total ionic strength adjustment buffer solution TISAB solution: same as example 1;
[0077] (2) preparation of fluoride ion standard stock solution (concentration is 1000mg / kg): same as example 1;
[0078] (3) preparation of fluoride ion standard test solution: with example 1;
[0079] (4) Sample analysis
[0080] Fluoride ion standard solution 1 and fluoride ion standard solution 2 were tested with a fluoride ion meter, and the electrode constant S was obtained as 59.57 according to their respective concentrations and potential values;
[0081] Weigh 6.5290g lithium bisfluorosulfonyl imide into a beaker, add 10mL total ionic strength adjustment buffer solution, then dilute to 50.0625g with distilled water, mix well to obtain a sample solution, the mass of the solution is m s , the solution potential E was measured with a fluoride ion meter 1 is 40.8mV;
[0082] Then add fluoride ion standard stock solution 0.1507g (m i ), after mixing evenly, measure t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com